Figure 5.
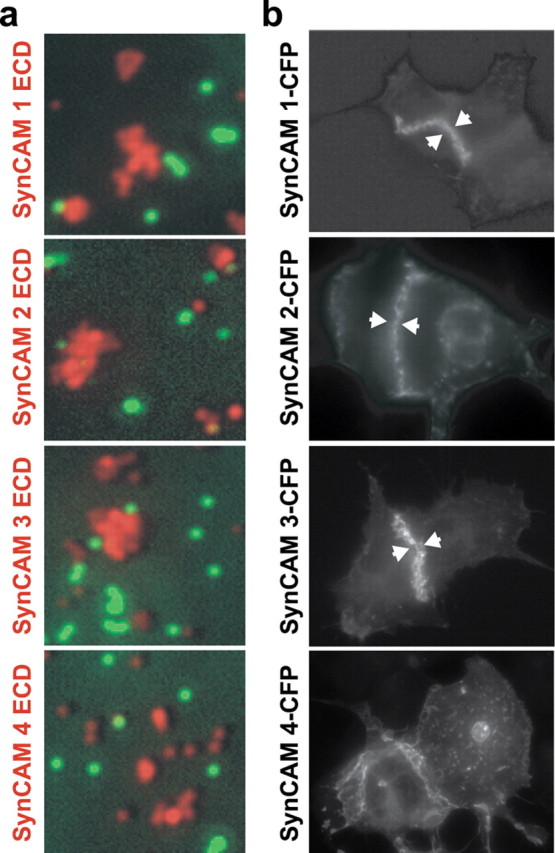
Homophilic cell adhesive interactions are mediated by SynCAMs 1, 2, and 3. a, The extracellular domains of SynCAM 1, 2, and 3 interact homophilically. Fluorescent beads coated with the purified, heterologously expressed extracellular domains of individual SynCAM proteins as indicated on the left of the panels (red) were mixed in the presence of negative control beads coated with IgG protein (green). Bound amounts of SynCAM proteins and control IgG were comparable (data not shown). Clustering of beads shown in red displaying SynCAM 1, 2, or 3 extracellular domains demonstrates their homophilic interaction, which was not observed for SynCAM 4. Negative control beads (green) were monodisperse and not found in SynCAM bead clusters. b, Homophilic SynCAM 1, 2, and 3 interactions occur in trans to mediate cell adhesion. HEK 293 cells were transfected with one of the full-length SynCAM 1, 2, 3, or 4 proteins. Proteins were tagged intracellularly with CFP, which did not interfere with plasma membrane sorting as confirmed by surface biotinylation (data not shown). Cells were analyzed by fluorescence microscopy, and localization of the indicated SynCAMs is shown in gray scale. Aggregation of SynCAM 1, 2, and 3 at sites of cell–cell contact is marked by arrowheads and demonstrates homophilic cell adhesion through trans-interactions of their extracellular domains. No homophilic cell adhesion was observed for SynCAM 4 in this assay, consistent with results in a.
