Figure 7.
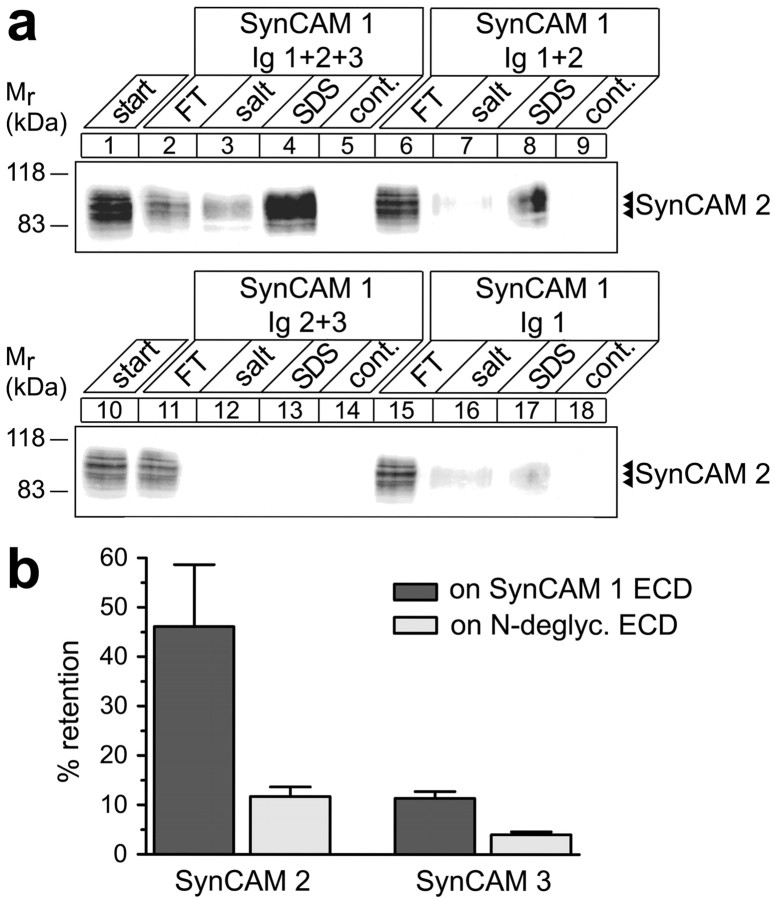
Heterophilic SynCAM binding is mediated by the first two Ig-like domains and is controlled by N-glycosylation. a, Mapping of SynCAM Ig-like domain interactions by affinity chromatography. Membrane proteins from adult rat forebrains were solubilized (lanes 1, 10) and loaded on beads containing equal amounts of covalently immobilized fusion proteins corresponding to the indicated SynCAM 1 Ig-like domains. The flow-through (FT) of each affinity chromatography was obtained, beads were washed, subsequently eluted with buffer containing high salt at 800 mm, and finally eluted with SDS. Eluates from beads containing only the immobilized fusion proteins served as negative controls for antibody cross-reactivity. Samples were analyzed by immunoblotting for SynCAM 2, which bound as strongly to the first three and first two Ig-like domains of SynCAM 1 (lanes 8 vs 4), but only weakly to its first Ig-like domain (lane 17), and not to either of the other Ig-like domains in combination (lane 13) or alone (data not shown). No binding of SynCAM 2 to beads containing control IgG was observed (data not shown). The numbers on the left indicate positions of molecular weight markers. Input and FT lanes contain 10% of the extract amount used for each affinity chromatography. b, Interactions of SynCAM 1 with SynCAM 2 are controlled by N-glycosylation of SynCAM 1 Ig-like domains. Membrane proteins from adult rat forebrains were solubilized and loaded on beads containing the full-length SynCAM 1 extracellular domain treated under native conditions either without or with PNGase F to remove N-linked carbohydrates. Binding was analyzed by quantitated immunoblotting. Control SynCAM 1 efficiently retained SynCAM 2, but natively N-deglycosylated SynCAM 1 exerted fourfold reduced binding. Consistent with a weaker interaction of SynCAM 3, it was retained approximately fourfold less on the control SynCAM 1 extracellular domain, and this retention was also reduced by deglycosylation of SynCAM 1. Error bars represent SDs (n = 3).