Figure 6.
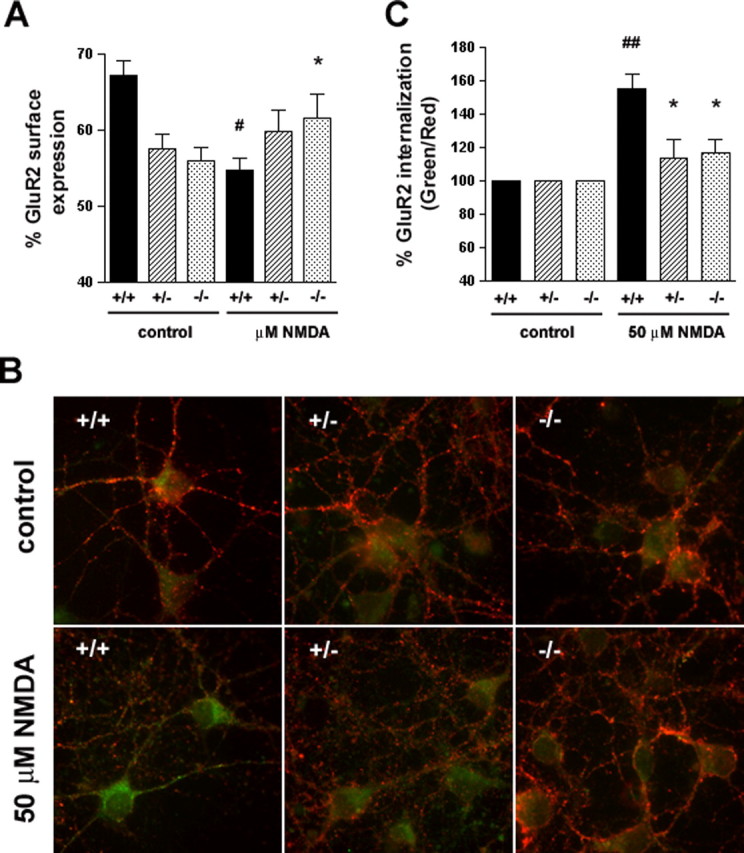
Reduced NMDA-induced AMPAR internalization in HIP1−/− neurons. A, HIP1 modulates NMDA-induced GluR2 trafficking in cortical neurons. Cortical neurons were established from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) littermates. After 12–15 DIV, the percentage of surface-expressed GluR2 was determined in a quantitative colorimetric assay in control and 100 μm NMDA-stimulated cultures under nonpermeant and permeant conditions. Results were averaged for each group in each of three independent experiments (#p < 0.05 for within-genotype, between-treatment comparison; *p < 0.05 for within-treatment, between-genotype comparison; t test). B, HIP1 modulates NMDA-induced GluR2 internalization in striatal neurons. Striatal neurons were cultured from wild-type (+/+), heterozygous (+/−), or homozygous (−/−) littermates and live-labeled with an Ab recognizing the extracellular domain of GluR2, followed by treatment with either control solution (ECS alone) or 50 μm NMDA, 10 μm glycine, and 5 μm strychnine for 5 min at 37°C. Cultures were washed and incubated for 20 min at 37°C to allow internalization. Surface GluR2 was then visualized using a red secondary Ab, whereas internalized GluR2 was labeled with a green secondary Ab. C, Internalization was quantified as the green-to-red signal ratio, which represents the ratio of the internalized GluR2 to the GluR2 remaining on the cell surface after different treatments (see Materials and Methods). Green-to-red ratios were averaged for each group in each of three independent experiments (##p < 0.005 for within-genotype, between-treatment comparison; *p < 0.05 for within-treatment, between-genotype comparison; t test). Error bars denote ±SEM.
