Figure 1.
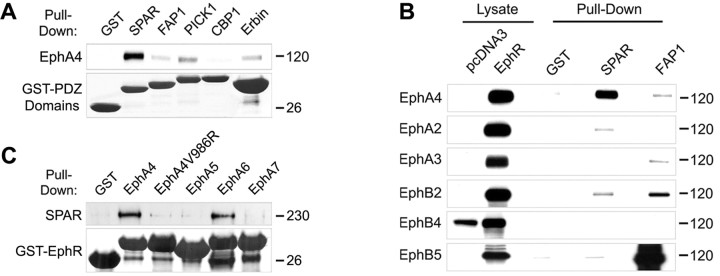
The C-terminal sequences of EphA4 and EphA6 bind the PDZ domain of the GTPase-activating protein SPAR. A, The PDZ domains of the indicated proteins, fused to GST, were used for pull-down assays with lysates of EphA4-transfected HEK 293T cells. Bound EphA4 was detected by immunoblotting and the GST-PDZ domain fusion proteins were detected by amido black staining. A lane between the CBP1 and Erbin lanes was digitally removed. B, Lysates from HEK 293T cells transfected with the indicated Eph receptors were used for pull-down assays with the PDZ domain of SPAR, and of FAP1 as a control, fused to GST. Bound Eph receptors were detected by immunoblotting. A lane between the lysate and pull-down lanes was digitally removed. C, The C-terminal tails of the indicated receptors, fused to GST, were used for pull-down assays with lysates of HEK 293T cells transfected with Myc-SPAR. SPAR bound to the GST fusion proteins was detected by immunoblotting with anti-Myc antibodies, and the GST fusion proteins were detected by amido black staining. In EphA4V986R, the C-terminal valine was mutated to arginine to inactivate the PDZ domain-binding motif.