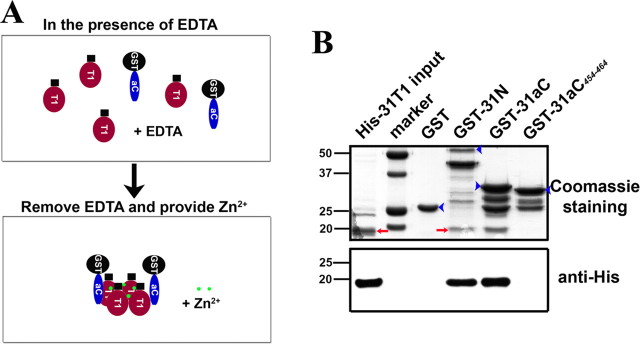
Figure 5.
Zinc regulates direct binding between purified His-31T1 and GST-31aC. GST and four other fusion proteins (His-31T1, GST-31N, GST-31aC, and GST-31aC454–464) were bacterially expressed and purified. In the normal pull-down buffer, GST-31N and GST-31aC did not efficiently precipitate His-31T1. Despite being evident in Western blotting, the precipitated His-31T1 was not detected by Coomassie staining. Therefore, we adopted a different pull-down strategy. A, Diagram of the procedure of pull-down assay. Purified His-31T1 and GST fusion proteins were first incubated overnight at 4°C in the presence of 1 mm EDTA to chelate Zn2+ to dissemble T1 tetramers into monomers. Then EDTA was dialyzed away and Zn2+ was added back to induce T1 monomers to reassemble into tetramers. B, GST-31N and GST-31aC, but not GST and GST-31aC454–464, efficiently precipitated His-31T1 in this pull-down assay. The precipitated His-31T1 was clearly detected in Coomassie staining. Identity of precipitated His-31T1 was confirmed with Western blot, in which only 10% of those in the top were loaded in each lane (bottom). For those purified fusion proteins, in addition to the main protein band, there are often a few smaller minor bands presumably attributable to protein degradation, which could occur even before the lysing and purification processes, because we have used protease inhibitors. The lower strong band in GST-31N may miss the T1-S1 linker, which does not compound our assay data. The lower bands in GST-31aC and GST-31aC454–464 are likely to be just GST. Because GST as a negative control included in our assay, these bands should not interfere with our experimental results as well. This pull-down experiment was repeated for three times. For Coomassie staining, 25% input of His-31T1 (the left lane) of the pull-down assay were loaded. Blue arrowheads, Full-length GST fusion proteins. Red arrows, His-31T1 bands. Numbers on the left, Molecular weights in kilodaltons.