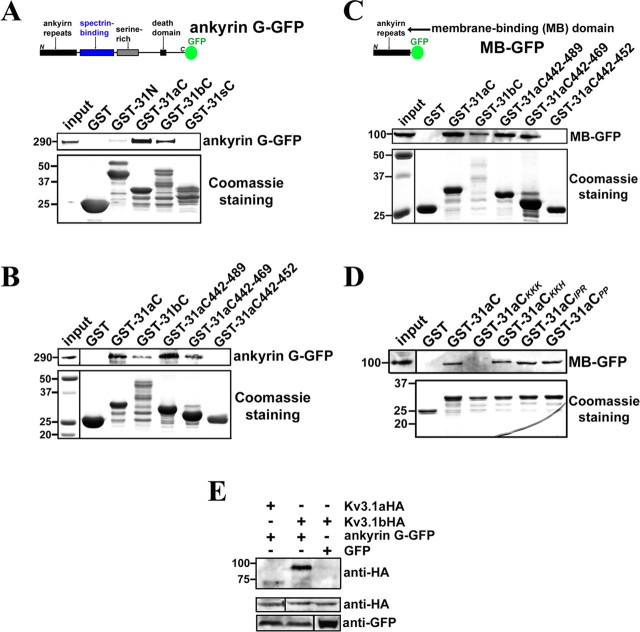
Figure 6.
The ankyrin-repeat domain of ankyrin G binds to the Kv3.1 ATM region. A, Ankyrin G-GFP was precipitated by purified GST-31aC and GST-31bC. The diagram shows the full-length ankyrin G-GFP construct, from the N to C terminus, containing the ankyrin repeat [also called membrane-binding (MB)] domain, spectrin-binding domain, serine-rich domain, and a death domain. GST, GST-31N, GST-31aC, GST-31bC, and GST-31sC were expressed in E. coli BL21 cells and purified with glutathione agarose beads. Then the beads coated with purified GST fusion proteins were further incubated with the supernatant of cell lysates from HEK293 cells transfected with ankyrin G-GFP for 3 d. The precipitated proteins were eluted, resolved in SDS-PAGE, and blotted with polyclonal anti-GFP antibody (top). The weak band in the GST-31N precipitant may result from an indirect interaction or a minor binding site. Five percent of the inputs of ankyrin G-GFP were loaded (left lane). The Coomassie staining shows 25% input of purified GST fusion proteins (bottom). B, Ankyrin G-GFP was precipitated by purified GST-31aC442–489 and GST-31aC442–469 but not GST-31aC442–452. The same experimental procedure as A was used. The left lane of Coomassie staining panel shows molecular weight standards. C, The ankyrin repeat or MB domain of ankyrin G binds to the ATM region. The top shows the diagram of MB–GFP. The experimental procedure was the same as that in B, except that the ankyrin G truncation MB–GFP was expressed in HEK293 cells. D, The lysine-rich motif but not the proline-rich motif is required for GST-31aC binding to MB–GFP. Four point mutants of GST-31aC were purified and used to pull down MB–GFP expressed in HEK293 cells. Only GST-31aCKKK failed to precipitate MB–GFP. E, Ankyrin G-GFP preferentially binds to Kv3.1bHA but not Kv3.1aHA in coimmunoprecipitation assay. Cotransfections were performed on HEK293 cells. Two days after transfection, the supernatant of cell lysates were incubated with polyclonal anti-GFP antibody and protein A agarose beads. The immunoprecipitants were blotted with rat monoclonal anti-HA antibody (top). Five percent of the inputs were loaded (middle and bottom). Numbers on the left, Molecular weights in kilodaltons.