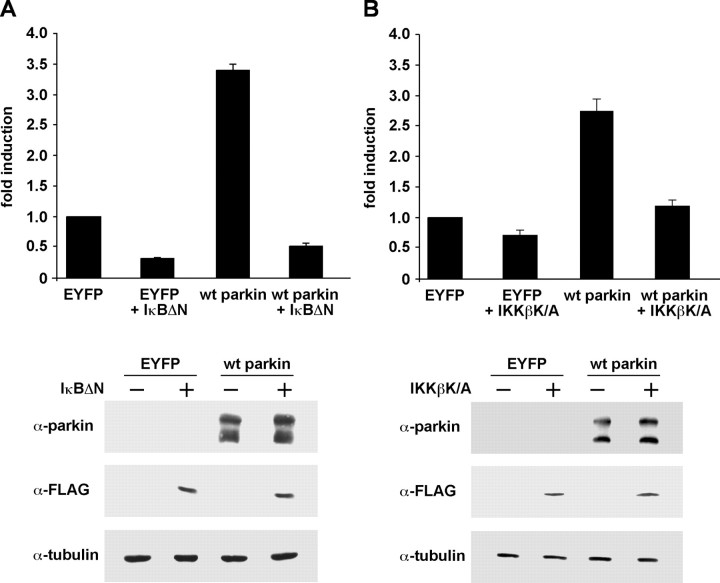
Figure 4.
The effect of parkin on NF-κB-dependent transcription can be antagonized by dominant-negative IκB or kinase-inactive IKKβ. A, HEK293T cells were cotransfected with NF-κB reporter plasmid, wt parkin, and the NF-κB super-repressor IκBΔN. At 24 h after transfection, cell lysates were analyzed for luciferase activity. Shown is the fold induction of luciferase activity compared with the EYFP control. As a control for protein expression, aliquots of the cell lysates were immunoblotted with the anti-parkin pAb hP1 and anti-FLAG mAb (bottom panels). α-Tubulin was used as a loading control. B, HEK293T cells were cotransfected with the NF-κB reporter plasmid, wt parkin, and the kinase-inactive IKKβ mutant IKKβ K/A. Luciferase assay and immunoblotting were performed as described in A.