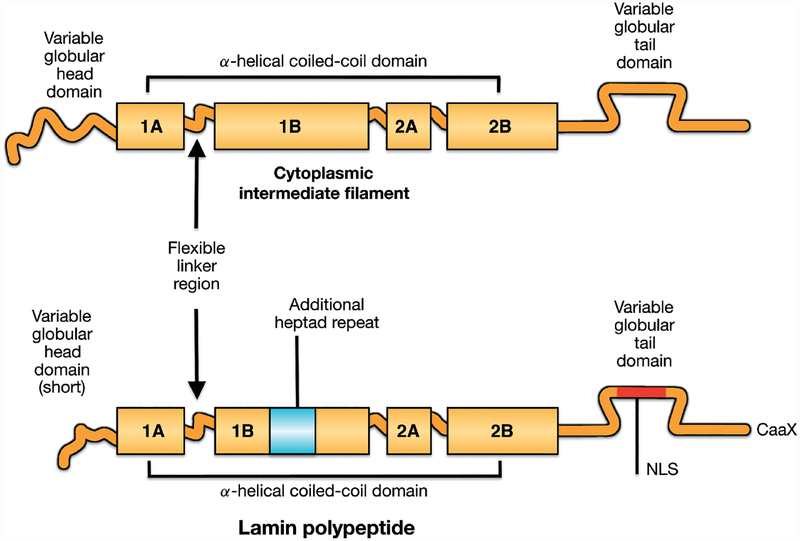
Figure 1.
Schematic diagrams showing vertebrate intermediate filament protein structures and differences between cytoplasmic intermediate filament proteins and nuclear lamins. All intermediate filament proteins, including lamins, have a conserved domain structure consisting a central α-helical coiled-coil rod domain consisting of four coiled coils (1A, 1B, 2A, 2B), based on heptad repeats, interrupted by flexible linker domains and a variable globular tail domain. Compared to vertebrate cytoplasmic intermediate filament proteins, lamins contain six additional heptad repeats (42 amino acids) in coil 1B, a nuclear localization signal (NLS) near an immunoglobulin-like fold domain in the carboxyl-terminal tail and, in most lamins, a CaaX motif at the carboxyl-terminus. Reproduced with permission from Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat Cell Biol 2004; 6: 1062–1067 [1].