Abstract
Social instability in primate groups has been used as a model to understand how social stress affects human populations. While it is well established that individual cercopithecines have different temperaments or personalities, little is known about how temperament mediates the experience of social instability in large, naturalistic groups. Here, we report findings from a study tracking a newly formed group of captive rhesus macaques (Macaca mulatta). We examine whether inter-individual differences in temperament during infancy affect physiological responses to new group formation years later, measured through hair cortisol nine months after the group was formed. Our results show that early-life measures of temperament characteristics predict later-life hypothalamic-pituitary-adrenal activity following new group formation, though not always in the directions we predicted. Individuals with higher blood cortisol concentrations in response to a novel stressor and lower blood cortisol concentrations following a Dexamethasone Suppression Test in infancy had lower hair cortisol values following new group formation later in life. Individuals characterized in infancy as more emotional or more active exhibited lower hair cortisol profiles nine months after group formation. We suggest that these two temperament characteristics, emotionality and activity, may represent two different mechanisms leading to low hair cortisol values. That is, the physiological measure of low hair cortisol may have two different meanings depending on temperament characteristics of the individual. Our results demonstrate that temperament and physiological responsiveness measures in infancy can predict individual responses to a new group formation years later.
Keywords: social instability, glucocorticoids, personality
Graphical Abstract
Introduction
Cercopithecine primate social groups are known for their stable cores of adult females and associated offspring (Silk, 2009). However, these groups can undergo periods of instability for myriad reasons, such as when a male immigrates into a group, a matriline becomes fragmented or declines in size, or two groups fuse (Alberts, Sapolsky, and Altmann 1992; Beisner, Jackson, Cameron, and McCowan, 2011; Brotcorne, Fuentes, Wandia, Beudels-Jamar, and Huynen, 2015; Samuels, Silk, and Altmann, 1987). Extreme social instability also occurs in captive cercopithecine primate groups (e.g., Anderson, Weladji, and Paré, 2016; Beisner et al., 2011; Gygax, Harley, and Kummer, 1997) and has been used as a model to understand the effects of social stress in humans (e.g., Beaulieu, Mboumba, Willaume, Kappeler, and Charpentier, 2014; Capitanio and Cole, 2015; Wilson, 2016).
It is well established from captive studies that individuals have different temperaments or personalities, and can respond in different ways to stressors based on temperament (e.g., Capitanio, Mendoza, Mason, Maninger, 2005; Rommeck, Capitanio, Strand, and McCowan, 2011; Hennessy, McCowan, Jiang, and Capitanio, 2014). Temperament is defined as stable traits of individuals manifested in consistent behavioral and associated physiological responses to circumstances encountered by the individual (Capitanio, 2011; Réale, Reader, Sol, McDougall, and Dingemanse, 2007). In primates, most work on temperament has used rhesus macaques (M. mulatta) as subjects (Freeman and Gosling, 2010). Previous studies of rhesus macaques have identified a number of health and social outcomes that differ for individuals based on temperament measures, such as infant weight gain, infant leukocyte sensitivity to cortisol, social relationships of yearlings, long-term and cross-situational behavior, and depressive-like behavior (Capitanio, 1999; Capitanio, Mendoza, and Cole, 2011; Hennessy et al., 2014; Hinde et al., 2015; Weinstein and Capitanio, 2008). Studies have also found links between infant temperament and HPA axis activity. For example, in captivity, rhesus macaques with more reactive temperaments also have higher hair cortisol concentrations, meaning they have experienced greater chronic activation of the HPA axis over the period of hair growth (Hamel et al., 2016). Captive juvenile rhesus macaques placed in novel peer groups respond differently depending on temperament: more reactive individuals have higher heart rates and higher levels of corticotropin and cortisol, hormones involved in the HPA axis (Suomi, 1997). Some primate temperament studies have examined responses to alteration in the social environment, but none to our knowledge has examined the responses to social instability in large social groups approximating wild populations in size and composition. Therefore, little is known about the ways in which individual temperament mediates the experience of social stress in these large, naturalistic groups.
Coping has been defined in various ways in the literature (Wechsler, 1995), and here we define it as individual responses to aversive stimuli that remove or attenuate deleterious physiological effects. One common view of coping puts individuals on a gradient from active coping style to passive coping style (Koolhaas et al., 1999), but here we use the term more broadly to consider other axes on which individuals may vary in their ability to avoid deleterious effects of stressors. For example, individuals can vary in emotional reactivity independent of variation in their active vs. passive coping styles, and this variation affects their susceptibility to depressive behavior and anxiety (Hennessy et al., 2014; Steimer, la Fleur, and Schulz, 1997). The effectiveness of different coping styles might depend on the context in which the stressor occurs, but within a given context such as captivity, differences among individuals in health, reproductive, and behavioral outcomes can indicate whether a particular coping strategy is effective. For example, among captive adult male rhesus macaques relocated from outdoor social groups to indoor housing, those who were more emotionally reactive in infancy exhibit more depressive-like behaviors, which indicates they have not coped as well with the stressor as other, less emotionally reactive, individuals have (Hennessy et al., 2014). Thus, in the context of captivity, certain temperament traits appear to index coping styles that can be viewed as comparatively better for dealing with the types of stressors an individual is likely to encounter. We might therefore expect individuals with different temperaments to experience different outcomes depending on the effectiveness of their coping styles during a period of social instability, such as the formation of a new group in captivity.
Here we examine whether inter-individual differences in early-life measures of biobehavioral organization, including temperament and HPA-axis organization, are associated with physiological and social responses to social instability caused by new group formation at the California National Primate Research Center (CNPRC). Our main outcome variable is hair cortisol concentration, which reflects long-term activity of the HPA axis (Davenport, Tiefenbacher, Lutz, Novak, and Meyer, 2006), a physiological system involved in the stress response.
The CNPRC’s ongoing BioBehavioral Assessment program assesses temperament traits via behavioral observation and HPA-axis organization via blood cortisol samples. This program has assessed the temperament and HPA-axis organization of many individual rhesus macaques, offering an opportunity to track how these traits affect patterns of individual behavior and physiological responses to various life events in captivity. Here, we use data from the BioBehavioral Assessment program to examine how these early-life measures of temperament and physiological responsiveness relate to HPA axis activity months to years later (range: 0.6 to 11 years after assessment), measured by hair cortisol concentrations before and after the formation of a new social group. We are interested in whether early-life traits predict later hair cortisol concentrations, and more specifically, whether the relationship between hair cortisol concentrations and temperament is altered during a period of social instability.
A number of temperament traits are measured in the BioBehavioral Assessment, and we narrow our focus here to four measures that previous work suggests might be particularly relevant to the experience of group formation. The first two are Activity and Emotionality scores thought to reflect different patterns of behavioral responsiveness to novel stressors (Capitanio et al., 2017). During BioBehavioral Assessment, infants are separated from their mothers and other familiar conspecifics for a 25-hr period. Initially, most individuals are behaviorally inhibited (showing low activity) and may overcome this inhibition as time passes. Some, but not all, infants are initially highly emotionally reactive, and emotionality generally decreases over time. Despite these broad patterns, individuals vary for each measure on both the first and second day of testing. In the context of the captive environment, higher Activity scores toward the end of the BioBehavioral Assessment demonstrate at least some adaptability to the novel stressor and are generally seen as indexing a more successful coping strategy, while higher Emotionality scores, particularly early during the BioBehavioral Assessment, indicate a more negative response to the stressors of separation and testing (Capitanio et al., 2017; Hennessy et al., 2014). If these traits are stable and occur generally in response to novel stressors, we expect both to affect coping with the stressor of group formation years later. The third temperament measure we examine is Nervous temperament, previously shown to lead to increased negative emotional responses to stressors and glucocorticoid desensitization (Capitanio et al., 2011). Nervous temperament might therefore also affect how individuals respond to the novel stressor of group formation later in life. Fourth, we examine Preference for Novelty, previously shown to predict high vs. low sociability in juveniles (Sclafani et al., 2016). Individual tendencies for high or low sociability might mediate differences in the experience of stress during social instability, as has been found in an experimental setting (e.g., Capitanio et al., 2008). Activity, Emotionality, Nervous temperament, and Preference for Novelty comprise the four early-life measures of behavioral responsiveness we examine as predictors of later-life responses to new group formation.
We also examine early-life variation in HPA-axis organization as a predictor of later-life responses to group formation. In addition to temperament measurements, the BioBehavioral Assessment program includes a series of blood samples over the course of testing to examine blood cortisol levels. We use two of these blood cortisol measurements, taken in infancy during BioBehavioral Assessment, as predictors of later-life responses to new group formation. High blood cortisol levels in response to novel stressors during BioBehavioral Assessment might predict higher hair cortisol concentrations following new group formation later in life. Similarly, more extreme declines in blood cortisol concentrations following Dexamethasone Suppression Tests (DST) might suggest increased sensitivity to down-regulating HPA axis mechanisms, and might predict low hair cortisol levels following new group formation.
We are interested in whether these measures of temperament and HPA axis organization have different relationships with hair cortisol values in the socially unstable period than they do in the socially stable period. We predict that high Activity, low Emotionality, low Nervous temperament, and high Preference for Novelty will be associated with lower hair cortisol concentrations following new group formation, because we believe these temperament traits reflect more successful coping with novel stressors. In addition, we predict individuals with low blood cortisol concentrations in infancy, both in Afternoon Response and DST, will have lower hair cortisol concentrations following group formation because they may continue to produce low levels of cortisol following social stressors.
Methods
Study Site and Subjects
The study was conducted at the CNPRC in Davis, CA. A new social group (NC 21-B) was formed on 7 May, 2012, as part of the CNPRC’s management practices for their outdoor breeding colony. The 0.2 ha field cage contained multiple perches and swings, and A-frame structures for protection from rain and wind. Behavioral management staff monitored the new group, which initially consisted of 111 individuals. Animals were sometimes temporarily or permanently removed from the field cage for medical treatment or management purposes. Our subjects included all individuals who were present in the field cage both times we collected hair samples for cortisol analysis and whose temperaments had previously been thoroughly profiled in infancy in the BioBehavioral Assessment program (N = 24). Data on individuals’ ages were obtained from CNPRC records (range: 0.9 to 11 years). We did not examine rank in relation to both BioBehavioral Assessment measurements and hair cortisol because we had rank data for only 17 of the 24 individuals, and because individuals in the new group formation came from different housing conditions, including indoor, solitary housing; consequently, rank could not meaningfully be compared across individuals under baseline conditions. A preliminary examination of the effects of rearing history indicated that none of the rearing conditions (indoor mother-rearing, rearing in small outdoor groups, or rearing in large outdoor field groups) significantly predicted hair cortisol values at nine months after the group formation (all P>0.75). For this reason, rearing history was not included in our subsequent analyses.
All procedures were approved by the Animal Care and Use Committee of the University of California, Davis. Hair samples were collected under IACUC-approved protocol #18090, and the BioBehavioral Assessments were conducted under various protocol numbers over the 16 years of the program, most recently IACUC-approved protocol #18588. All procedures adhered to the legal requirements of the United States of America, and to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
BioBehavioral Assessment Data Collection
We used data collected from CNPRC’s BioBehavioral Assessment program, an ongoing, long-term project on behavioral and physiological responsiveness that has been described in detail elsewhere (see, e.g., Capitanio, 2017; Capitanio et al., 2005; Capitanio, Mason, Mendoza, Del Rosso, and Roberts, 2006; Golub, Hogrefe, Widaman, and Capitanio, 2009). In general, infants approximately 3-4 mos old (range for present sample: 93-126 days old) are removed from familiar social partners, including their mothers, and introduced to a novel indoor testing environment where they are housed individually for approximately 25 hours before being returned to their mothers and then to their home housing. Behavioral and physiological tests are conducted over the 25-h period to assess the individuals’ responsiveness to the novel testing conditions, and to particular stimuli. The current study uses four types of measures from BioBehavioral Assessment as predictors of later-life hair cortisol concentrations (Table 1). All BioBehavioral Assessment data are provided in the supplementary materials (Table S1).
Table 1.
BioBehavioral Assessment (BBA) measures and other variables considered as predictors of hair cortisol.
| Predictor name | Description |
|---|---|
| Afternoon Response | BBA blood cortisol value for afternoon response (7 hr into testing) |
| DST | BBA blood cortisol value for dexamethasone suppression test |
| Day 1 Activity | BBA activity z-score for day 1 of testing |
| Day 2 Activity | BBA activity z-score for day 2 of testing |
| Day 1 Emotionality | BBA emotionality z-score for day 1 of testing |
| Day 2 Emotionality | BBA emotionality z-score for day 2 of testing |
| Nervous | BBA z-score for nervous temperament |
| Preference for Novelty | BBA score for proportion of time looking at novel image across all preferential look test trials |
| Age | individual’s age in days when hair sample was collected |
| Sex | individual’s sex (0 = male, 1 = female) |
Activity and Emotionality:
Activity and Emotionality scores were assigned for Day 1 and Day 2 of the 25-h assessment period based on focal behavioral observations conducted by trained observers with annual inter-observer reliabilities greater than 85%. Five-min focal observations were conducted beginning approximately 15 min after the animals arrived in the testing suite (Day 1), and again 22 hrs later (Day 2). We consider both Day 1 and Day 2 measures here, because either immediate responses (Day 1) or slightly more long-term adaptation (Day 2) to BioBehavioral Assessment might be relevant to how the individual copes with the novel stressor of group formation. Activity and Emotionality scales were identified through exploratory and confirmatory factor analyses of the various behaviors scored by observers (described in Golub et al., 2009). “Activity” included the following quantitatively measured behaviors: proportion of total time spent locomoting, proportion of total time spent not hanging from the top/side of cage, rate of environmental exploration, a dichotomous variable for whether individuals ate food or not, a dichotomous variable for whether individuals drank water or not; and a dichotomous variable for whether individuals crouched or not. “Emotionality” included the following quantitatively measured behaviors: rate of cooing, rate of barking, a dichotomous variable for whether individuals scratched or not; a dichotomous variable for whether individuals displayed threats or not; and a dichotomous variable for whether individuals lipsmacked or not. Each of the four scales was z-scored following procedures established in Golub et al. (2009), and these measures were used as predictors in the analysis.
Nervous Temperament:
At the end of the 25-hr assessment period, observers rated each animal on a scale of 1 to 7 for each of 16 traits. Previous work from the BioBehavioral Assessment program has identified four temperament scales in rhesus macaques using exploratory and confirmatory factor analyses of these 16 traits (described in Golub et al., 2009). These scales are Vigilant, Gentle, Confident, and Nervous temperaments. We focus here on one of these scales, Nervous temperament, because previous work suggests it mediates negative emotional responses to stressors, such as the new group formation examined here (Capitanio et al., 2011). Individuals were rated for how nervous, fearful, timid, calm, and confident (the last two items were reverse scored) they were. We used z-scores for Nervous temperament within each year’s cohort of testing subjects as predictors in this study’s analysis.
Preference for Novelty:
This measure was included in our analysis because previous work has linked Preference for Novelty to high vs. low sociability in juvenile rhesus macaques (Sclafani et al., 2016), and sociability might mediate individual perceptions of the new group formation as stressful. Preference for Novelty was assessed approximately 2.5 hours into BioBehavioral Assessment using a visual paired comparison (i.e., preferential looking) test with pictures of unfamiliar animals as stimuli. Animals were given seven “problems,” each lasting 51 sec, and shown on a video monitor (see Sclafani et al., 2016, for more details and links to the stimuli). For each problem, individuals were shown a blank white screen (5 sec), followed by a pair of identical images displayed on the screen during a familiarization period (20 sec). Following another blank screen (5 sec), a pair of images, one now-familiar and one novel, randomly placed to the right or left side of the screen was displayed for 8 sec, after which the animals saw a blank white screen (5 sec), followed by the same pair of images swapped to opposite sides of the screen (8 sec). Animals were filmed and observers scored the duration of time spent looking at the images. For some trials individuals were not scored because they were not facing the screen during the familiarization period. A mean value for each individual was taken across all of the problems that individuals completed, and was computed as the proportion of total looking time that the animal looked at the novel stimuli (i.e., (duration of looking at novel)/(duration of looking at novel + familiar).
Blood Cortisol Concentrations
We used data from two blood samples taken during BioBehavioral Assessment and assayed to measure cortisol concentrations. One sample was drawn on Day 1 approximately 7 hr into testing (1600 h), and thus reflects the animal’s “Afternoon Response” to the testing experience: presumably, higher cortisol concentrations at this time point reflect the continued experience of stress. Animals were then given a dexamethasone injection (500 ug/kg), which suppresses cortisol production, and another blood sample was drawn the following morning, approximately 23.5 h after arrival for testing (0830 h). Cortisol responses to the Dexamethasone Suppression Test reflect the functioning of the HPA axis’s negative feedback mechanism. These measures are described in more detail in Capitanio et al. (2005).
Hair Sample Collection
We collected hair samples from the subjects on 15 May, 2012 (which was 8 days after the group formation), and again nine months later on 12 Feb, 2013, during routine health checks conducted by CNPRC staff. Management staff sedated animals using weight-specific doses of ketamine and shaved hair from the inner thigh of each individual present in the enclosure. We placed each hair sample in an aluminum foil pouch and labeled the sample with the individual’s unique identification number, the date of collection, and the enclosure number. Foil pouches were then sealed and stored at room temperature until laboratory processing for assay.
Hair Sample Processing
We processed hair samples following a modification of Davenport et al., (2006). We cleaned and processed hair samples to extract cortisol and reconstituted it in a liquid substrate that could be assayed. To wash the hair, we weighed out 100 mg of each sample (or the maximum weight possible if the sample contained less than 100 mg). We then placed these 100 mg-aliquots in a labeled 15 ml conical tube with 5 ml isopropanol and gently inverted to remove external contaminants. The isopropanol was then poured out and the wash was repeated with another 5 ml isopropanol. Once this was completed, we moved the hair to a closed petri dish to dry for at least five days.
We then weighed 35-mg aliquots of each sample and placed them in labeled microcentrifuge tubes. Within each tube, we minced hair using nail scissors and ground the minced hair in a Retsch Ball Mill by adding two 7-mm steel balls to each tube and placing tubes in 5-slot welled jars. Samples were ground for 10 min at 30 hz. True weights of each sample were calculated by subtracting the empty tube weight from the sample-plus-tube weight after grinding.
We extracted cortisol from powdered hair by adding 1ml methanol to each tube and placing tubes in a rotator overnight (18-24 hrs). We pelleted powdered hair in a microcentrifuge for five min and siphoned 0.6ml methanol from the top into a new labeled microcentrifuge tube. The methanol in each tube then dried under a stream of air, leaving a dry film containing cortisol at the bottom of each tube. To assay the cortisol, we added 0.4 ml of diluent to each tube, using buffer solution from commercially available assay kits (Salimetrics, State College, PA). Each sample was vortexed for 20 sec before storing in a freezer at −80 degrees Celsius until assay.
We then estimated cortisol concentrations in duplicate using commercially available Salimetrics Cortisol Assay kits. Finally, we converted these concentrations to pg/mg hair to allow comparison among slightly different weights of hair in each sample.
This process produced a series of two hair cortisol concentrations for each of 24 individuals: hair cortisol values from the hair samples collected soon after group formation in May 2012 (“Baseline Hair Cortisol”) and hair cortisol values from the hair samples collected nine months later in February 2013 (“9-mo Hair Cortisol”). We limit our sample to individuals present at both hair sample collections, as many individuals were removed because they suffered or caused frequent trauma during the nine months between hair sample collections. We consider the first set of samples “Baseline” because, although they were collected eight days after the new group formation, hair cortisol reflects a long period of time, hair growth begins beneath the surface of the skin, and hairs grow asynchronously (Fourie and Bernstein, 2011; Meyer and Novak, 2012). Thus, any growth during this time would have had minimal impact on cortisol concentrations.
Post-hoc Examination of Trauma
To help interpret our results, we also examined which individuals had to be removed from the group because they suffered severe or recurrent physical trauma during the nine months following the new group formation. Trauma was assessed by behavioral management staff independently from researchers, as part of ongoing management practices. We coded permanent removal due to trauma as a dichotomous variable based on whether individuals were permanently removed in the nine months following new group formation.
Statistical Analyses
We used log likelihood-ratio tests to compare three mixed-effect models fitted to hair cortisol values with the lme function from the nlme package in R, using the maximum likelihood method (Pinheiro, Bates, DebRoy, Sarkar, and R Core Team, 2018). All predictors are listed in Table 1. The Null Model included fixed effects for change in cortisol at nine months, sex, and age, and random effects for individual. The Baseline Effects Model also included effects for the BioBehavioral Assessment measures, assuming those effects were the same at Baseline and at Nine Months. The Nine-Month Effects Model allowed BioBehavioral Assessment effects to vary at nine months, entertaining the possibility that these measures affect hair cortisol differently during periods of social instability. We used the anova function in R to compare models (R Core Team, 2016). Comparing these three models allowed us to ask whether knowing an individual’s temperament tells us something meaningful about its HPA activity in general (Baseline Effects Model), or their HPA activity following a novel social stressor more specifically (Nine-Month Effects Model).
Two individuals had missing data for one BioBehavioral Assessment measurement each. We performed conditional mean imputation to impute the expected values in each of those fields using sex and BioBehavioral Assessment variables from a broader dataset of 47 individuals. We log-transformed age, Afternoon Response, and DST to ensure Gaussian distributions.
Results
The Nine Months Effects Model fit the data significantly better than the Baseline Effects Model, which itself was significantly better than the Null Model (Table 2). Thus, BioBehavioral Assessment measures provided useful information in predicting hair cortisol values and they had effects during the period of social instability that they did not have under stable social conditions.
Table 2.
Description and results of model comparison for the three models considered.
| Model | Predictors | Intraclass Correlation Coefficient | log likelihood |
|---|---|---|---|
| Null | Sex, age, shared 9-month effect, random intercepts for individuals | 0.23 | 32.94 |
| Baseline Effects | Same as Null, along with BioBehavioral Assessment measures | 4.16*10^-10 | 43.07 |
| Nine Months Effects | Same as Baseline Effects, along with interaction of BioBehavioral Assessment measures and 9-month effect | 0.24 | 55.44 |
log likelihood ratio comparisons: Baseline-vs-Null: 20.26, p<0.01; Nine-Months-vs-Baseline: 24.75, p<0.002
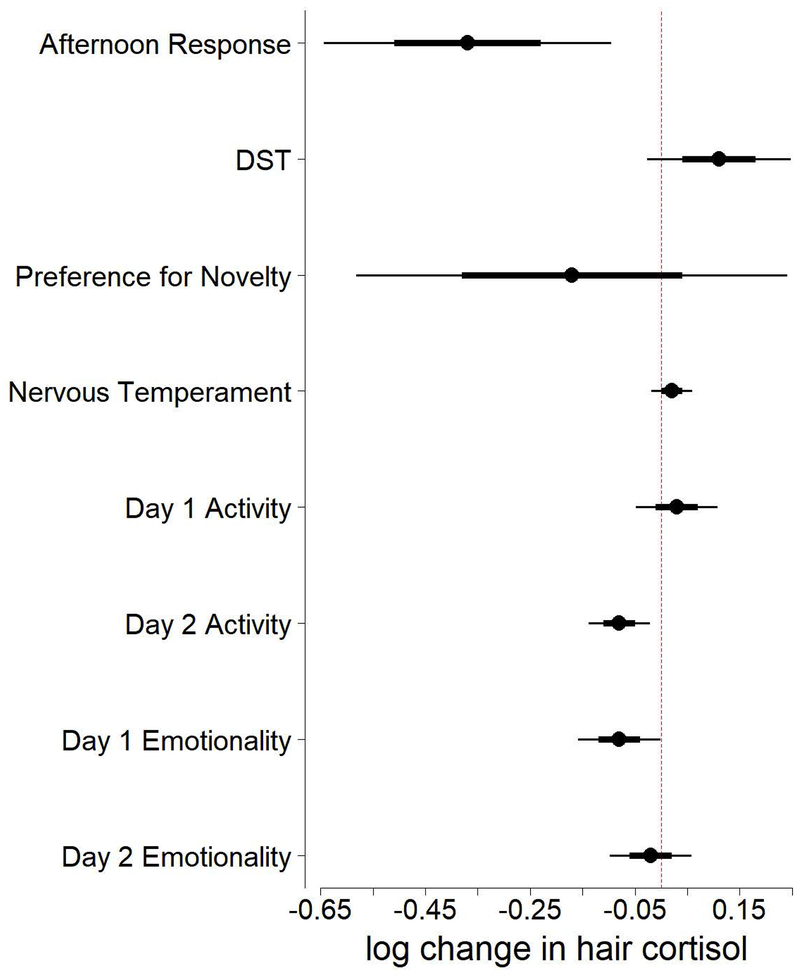
We can examine the coefficients relative to their standard errors (Table 3, Figure 1) to infer which BioBehavioral Assessment measures are better supported as influencing hair cortisol values at Nine Months and examine the directionality of their effects. These coefficients indicate how each variable affects an individual’s change from Baseline Hair Cortisol to Nine Month Hair Cortisol.
Table 3.
Coefficients for effects of BioBehavioral Assessment measures on change in hair cortisol at nine months after the group formation, from Nine-Months Effects model
| Nine-month effect of: | Coefficient | Standard Error |
|---|---|---|
| Afternoon Response | −0.37 | 0.14 |
| Dexamethasone Suppression Test | 0.11 | 0.07 |
| Preference for Novelty | −0.17 | 0.21 |
| Nervous Temperament | 0.02 | 0.02 |
| Day 1 Activity | 0.03 | 0.04 |
| Day 2 Activity | −0.08 | 0.03 |
| Day 1 Emotionality | −0.08 | 0.04 |
| Day 2 Emotionality | −0.02 | 0.04 |
Figure1:
Nine Months Effects model coefficients (circles), standard errors (heavy black lines), and 95% confidence intervals (narrow black lines). Coefficients indicate model-estimated change in hair cortisol concentrations following group formation for each unit increase in BioBehavioral Assessment measures. Dashed red line indicates estimated change of 0. Note that because BioBehavioral Assessment measures are in different units, the relative effects of coefficients are not directly comparable in this figure.
Afternoon Response, DST, Day 2 Activity, and Day 1 Emotionality each have a fairly large coefficient relative to standard error, but not all these relationships were in the direction we predicted (Table 4). As predicted, individuals with higher blood cortisol values following the Dexamethasone Suppression Test in infancy also had higher hair cortisol values nine months after the new group formation later in life. Also as predicted, individuals who were more active during the stressor of BioBehavioral Assessment in infancy had lower hair cortisol values after the new group formation. Contrary to our predictions, individuals with higher Afternoon Responses in their blood cortisol values during BioBehavioral Assessment had lower hair cortisol values following the new group formation. Also contrary to our predictions, individuals who responded more emotionally to the stressor of BioBehavioral Assessment in infancy also had lower hair cortisol values following the new group formation.
Table 4.
Predictions compared to observed effects of four BioBehavioral measures from infancy on hair cortisol values 9 months after group formation later in life.
| Nine-month effect of: | Hypothesis | Predicted association with hair cortisol after group formation | Observed association with hair cortisol after group formation | Support? |
|---|---|---|---|---|
| Afternoon Response | Greater short-term HPA axis response to stressor in infancy is associated with greater long-term HPA axis response to stressor of new group formation. | + | − | No |
| Dexamethasone Suppression Test | Greater sensitivity to downregulating mechanisms of HPA axis in infancy is associated with greater downregulation of HPA axis later in life. | + | + | Yes |
| Emotionality | Individuals who responded more emotionally to stressor in infancy are more sensitive to stressor of new group formation later in life, leading to higher hair cortisol levels. | + | − (Day 1) | No |
| Activity | Individuals who overcame behavioral inhibition during stressor in infancy adapt more quickly to new group formation later in life, leading to lower hair cortisol levels. | − | − (Day 2) | Yes |
| Nervous temperament | Individuals characterized in infancy as more nervous are more sensitive to the stressor of new group formation later in life, leading to higher hair cortisol levels. | + | 0 | No |
| Preference for Novelty | Greater preference for novelty has been linked to greater sociability, which in turn may help individuals better manage the stressor of new group formation later in life, leading to lower hair cortisol levels. | − | 0 | No |
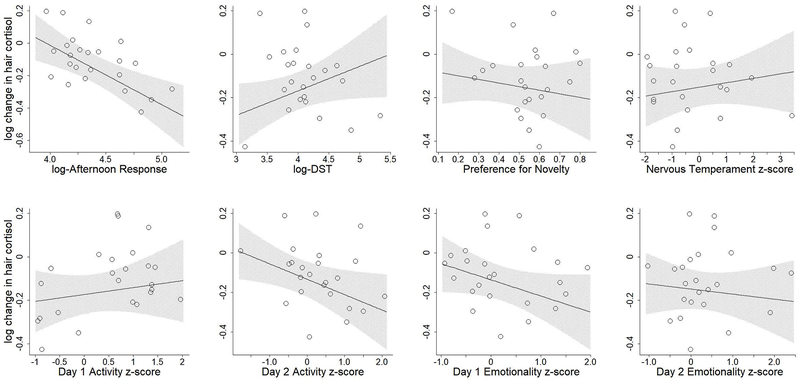
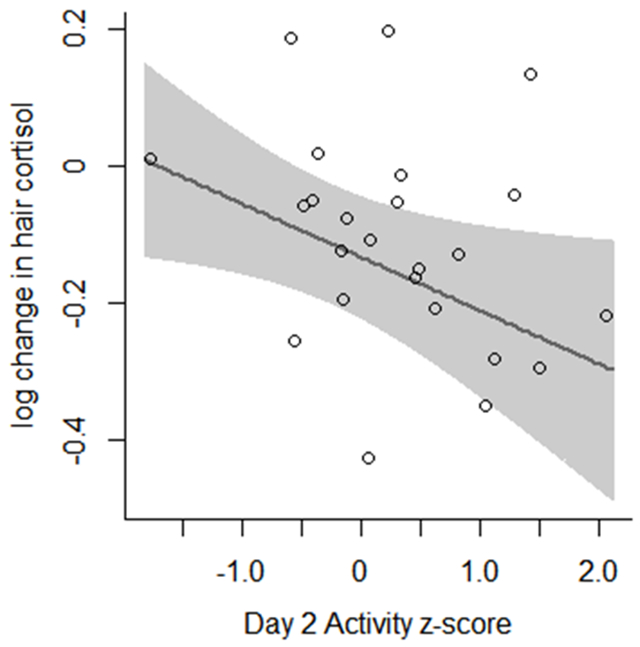
To visualize the data, we plotted the model-estimated change in hair cortisol (delta-log-cortisol) for females across different values of the BioBehavioral Assessment measures, assuming average values for the other BioBehavioral Assessment measures and age (Figure 2). Note that because these plots assume other BioBehavioral Assessment measures and age are average, the lines are not directly comparable to the datapoints shown in each plot.
Figure2:
Nine Months Effects model-estimated change in hair cortisol concentrations relative to BioBehavioral Assessment measurements. Open circles represent individual datapoints. Line indicates mean expected change in hair cortisol concentration, holding other parameters constant. Shaded region indicates 95% confidence interval for mean. Note that estimated means were calculated assuming the individual was female and had average values for other parameters, and therefore raw datapoints are not directly comparable to estimated means.
Post-hoc Examination of Removal Due to Trauma
During the BioBehavioral Assessment, most infants are not highly emotionally reactive, and emotionality also tends to decrease from Day 1 to Day 2. Greater emotionality therefore suggests a more extreme negative response to the stressor of BBA testing. On the other hand, individuals that manage to overcome their initial (Day 1) behavioral inhibition more quickly, becoming more active on Day 2, have demonstrably adapted to the stressor to some extent. In addition, greater Day 1 Emotionality is linked to longer-term deleterious outcomes in captive rhesus macaques (e.g., Hennessy et al., 2014), while greater Day 2 Activity is linked to more positive outcomes (e.g., K. Hinde, unpublished data; see Discussion). High initial (i.e., Day 1) emotional reactivity is therefore generally interpreted in the research produced by the BioBehavioral Assessment program as a negative reaction to the stressor, while greater activity on Day 2 is interpreted as reflecting more successful coping. Thus, we initially predicted that high Emotionality would lead to higher hair cortisol values and high Activity would lead to low hair cortisol values following the new group formation; our results for Emotionality, however, were in the opposite direction. There are several possible explanations for this. Perhaps both high-Activity and high-Emotionality individuals adapted well to this particular stressor, despite previous work suggesting high Emotionality or low Activity are linked to poorer outcomes (Hennessy et al., 2014, K. Hinde, unpublished data). Perhaps neither high-Emotionality nor high-Activity individuals adapted well, leading to HPA axis dysregulation as has been seen in some other studies of social instability (e.g., Capitanio, Mendoza, Lerche, and Mason, 1998). Or perhaps both frameworks are relevant here, with high Activity leading to low hair cortisol via adaptive coping and high Emotionality leading to low hair cortisol due to HPA axis dysregulation. While the available data for this group formation cannot conclusively distinguish these hypotheses, we were able to conduct a post-hoc analysis to look for differences in how highly active and highly emotional individuals fared following the new group formation, which might indicate differences in how well they coped with the stressor. We focused on the experience of severe or recurrent physical trauma during the social instability following new group formation as an indicator of coping with the new group formation. These injuries could be severe, and individuals who managed to avoid them presumably coped with the social instability in ways that those who did suffer serious injuries did not.
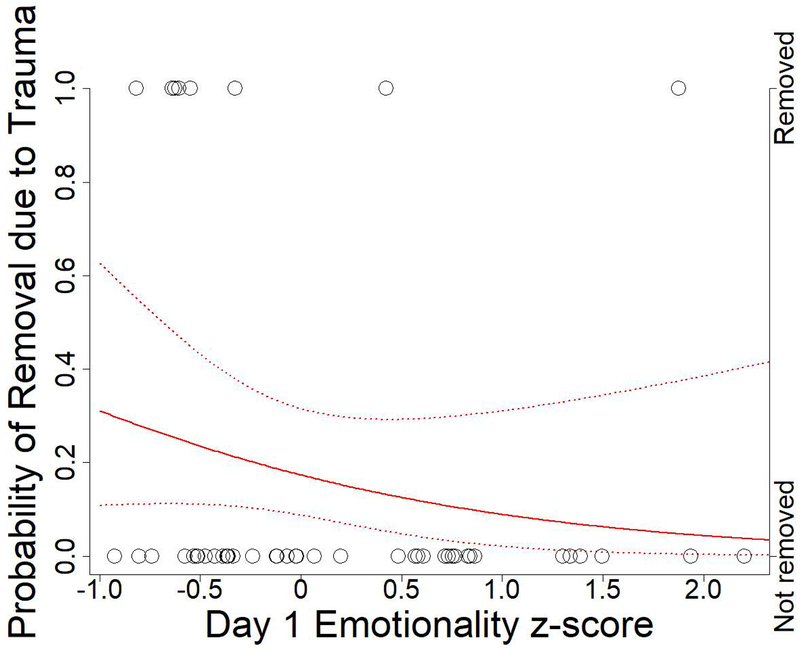
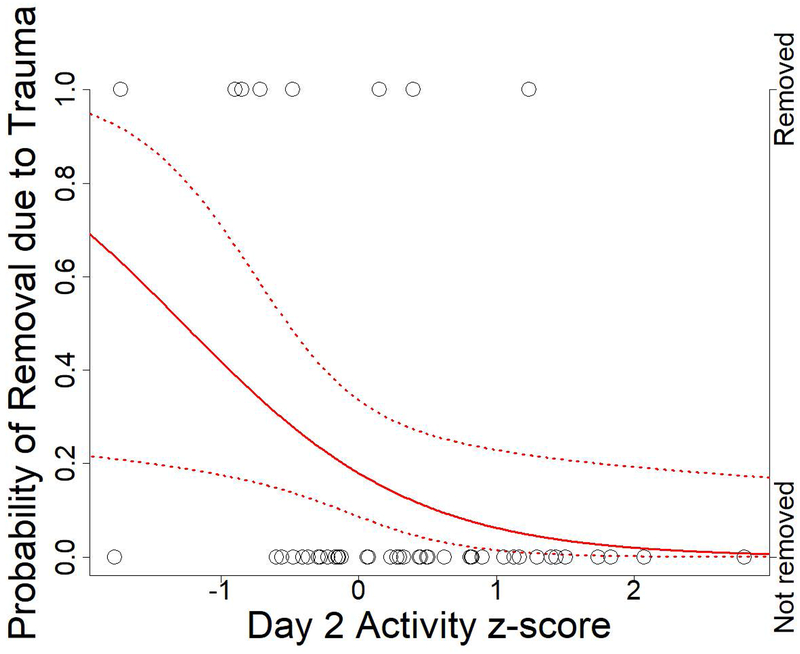
Therefore, for the broader sample of 47 individuals with BioBehavioral Assessment data present at the beginning of the new group formation, we also examined Day 1 Emotionality and Day 2 Activity scores in relation to removal from the group due to physical trauma. As aggression is common among rhesus macaques, particularly during periods of social instability, many individuals suffered socially inflicted trauma in the aftermath of the new group formation. Individuals requiring veterinary treatment for this trauma were removed from the group and treated. Assessment for veterinary treatment was conducted by veterinary staff independently of the researchers, following established protocols at the CNPRC. Some of the individuals removed for treatment were then returned to the group, but others suffered such severe or recurrent trauma that they had to be permanently relocated for their own safety. Using removal data obtained from CNPRC records, we fitted logistic regression models in R with the glm function (R Core Team, 2016). High Day 1 Emotionality did not reduce the likelihood of removal due to trauma during the nine-month period immediately following group formation (Figure 3, ß = −0.77, SE = 0.61, P = 0.2), but high Day 2 Activity did, and the estimated effect in the model was larger (Figure 4, ß = −1.19, SE = 0.55, P = 0.03). Thus, high Day 2 Activity individuals coped with trauma, either by avoiding more severe trauma or by avoiding recurrences of trauma, in a way that high Day 1 Emotionality individuals did not.
Figure3 :
Probability of removal due to trauma by Day 1 Emotionality. Open circles represent whether an individual was removed during the 9-month study period after group formation. Solid red line indicates estimated probability of removal due to trauma for varying Day 1 Emotionality scores. Red dotted lines indicate 95% confidence intervals for estimated probabilities.
Figure 4:
Probability of removal due to trauma by Day 2 Activity. Open circles represent whether an individual was removed during the 9-month study period after group formation. Solid red line indicates estimated probability of removal due to trauma for varying Day 2 Activity scores. Red dotted lines indicate 95% confidence intervals for estimated probabilities.
Discussion
Early-life measures of temperament and physiological responsiveness are important in predicting individual changes in hair cortisol concentrations during a period of social instability later in life. Four measures considered in our study are particularly salient in predicting these changes in cortisol levels. Two are behavioral temperament metrics, Activity and Emotionality. The other two are blood cortisol measurements, both the Afternoon Response to BioBehavioral Assessment and the response to the Dexamethasone Suppression Test. These measures were not all associated with hair cortisol in the ways we predicted, however, and below we discuss possible explanations for these results. We failed to find evidence that Preference for Novelty or Nervous Temperament influenced individuals’ hair cortisol values following the new group formation. Below, we consider the implications of our results in terms of how temperament might influence cortisol levels.
Activity and Emotionality
We predicted that higher Emotionality and Activity would have positive and negative effects, respectively, on hair cortisol concentrations following the group formation. However, we found that both were associated with lower hair cortisol values. During BioBehavioral Assessment, Emotionality and Activity may be interpreted as reflecting coping responses to the immediate (Day 1) and longer-term (Day 2) relocation and separation. Immediately upon relocation to the BioBehavioral Assessment room on Day 1, individuals typically exhibit low rates of the behaviors encompassed in Activity scores (Capitanio, 2017), but by Day 2, as animals become more comfortable in the situation, Activity levels generally increase (e.g., locomoting and eating more, while spending less time hanging on the side of the cage). In contrast, behaviors associated with Emotionality are typically higher on Day 1 compared to Day 2 (although we note that the modal rate of vocalization on Day 1 is zero). Generally, then, greater Day 1 Emotionality means that the individual is highly emotionally reactive, which is interpreted as reflecting poor coping during BioBehavioral Assessment. Greater Day 2 Activity means that the individual has overcome any initial behavioral inhibition, which is interpreted as reflecting successful coping. In fact, follow-up studies that we have conducted support this interpretation: Hennessy et al. (2014) found that high Day 1 Emotionality during BBA was significantly associated in adulthood with a greater likelihood of displaying depressive behavior upon removal from the animal’s familiar social group. Similarly, in an analysis of reproductive performance among females, individuals with high Day 2 Activity showed the typical pattern of reproduction, having their first viable birth at 3 or 4 years of age, while animals with low Day 2 Activity did not have their first viable offspring until 5 years of age or later (K. Hinde, unpublished data). In short, Activity and Emotionality scores measured in infancy have been shown to map on to different outcomes later in life that suggest, at least for stressors encountered in the captive environment, the coping style of highly active individuals may be more adaptive than the coping style of highly emotionally reactive individuals. It is therefore surprising that both high Day 1 Emotionality and high Day 2 Activity were associated with lower hair cortisol levels following new group formation. These two measures are not significantly correlated (Pearson’s r = −0.08, n=24, P=0.7), meaning that the individuals with high Day 1 Emotionality are not the same individuals with high Day 2 Activity. We expected lower cortisol values in high Day 2 Activity individuals, because these individuals adapted well to the stressor of BioBehavioral Assessment and might therefore be expected to adapt well to the new group formation later in life, and found support for this hypothesis. Our post-hoc analysis provides further support for the idea that more active individuals coped with the new group formation in ways other individuals did not: more active individuals were less likely to require permanent removal due to trauma, meaning that they avoided severe, recurrent injuries from their groupmates. Importantly, the relationship between activity in infancy and later hair cortisol concentrations is not likely a result of the fact that physical activity is metabolically associated with greater cortisol production (Sapolsky, Romero, and Munck, 2000): our finding that Day 2 Activity is associated with lower 9 month Hair Cortisol demonstrates an effect in opposition to the expected metabolic influence of physical activity levels on cortisol production. Taken together, our results and previous research are consistent with the hypothesis that activity is a coping style that allows captive rhesus macaques to successfully adapt to novel situations, leading to more typical patterns of reproduction (K. Hinde, unpublished data) or less severe injuries in periods of social instability, as shown here.
Emotional reactivity has been linked to poor outcomes in rhesus macaques that have been relocated indoors (Hennessy et al., 2014). Why, then, do highly emotional individuals in our sample have lower hair cortisol following the new group formation? There are several possible explanations. First, perhaps these individuals cope well with the new group formation, as more active individuals do, leading to lower cortisol values nine months later. In making our initial predictions, we used the simplistic theoretical framework that better coping leads to reduced experience of stress, and in turn, lower cortisol levels. However, as discussed above, highly emotional individuals are known to adapt poorly to a different novel stressor, social isolation (Hennessy et al., 2014). Future research will be necessary to examine how well highly emotional individuals cope with the specific stressor of group formation. However, we should not ignore alternative explanations based on other factors that are known to alter HPA activity.
We therefore offer an alternative interpretation based on previous work, although we note that this explanation is a hypothesis and further research will be necessary to test it. We initially predicted that greater emotionality would lead to higher hair cortisol concentrations following the new group formation because highly emotional captive rhesus macaques tend to have negative outcomes indicative of poorer coping with novel stressors. However, regulatory mechanisms of the HPA axis that normally function well in some individuals may become dysregulated in others following chronic activation of the HPA axis (Beehner and Bergman, 2017; McEwen and Wingfield, 2003; Romero, Dickens, and Cyr, 2009). Two individuals might therefore have low cortisol levels compared to conspecifics, but for different underlying reasons: one because its successful coping strategy allows it to maintain low cortisol levels, and the other, because its poorer coping style has led to such chronically high levels of cortisol that its HPA axis has become dysregulated. If animals that are highly emotionally reactive as infants continue to respond emotionally to subsequent stressors, it could lead to chronic activation of the HPA axis. In humans, low hair cortisol levels have been shown for individuals experiencing chronic stress, such as major depression disorder (Pochigaeva et al., 2017), post-traumatic stress disorder (Steudte et al., 2013), or suicide attempts (Melhem et al., 2017), and low plasma cortisol levels following Dexamethasone Suppression Tests have been shown in individuals with bipolar disorder (Maripuu, Wikgren, Karling, Adolfsson, and Norrback, 2017). A similar effect has been demonstrated in rhesus macaques subjected to chronically stressful social conditions (Capitanio, et al., 1998). This is presumably because chronic stress alters sensitivity of the HPA axis, causing down-regulation of cortisol production. For example, Maripuu et al. (2017) found decreased blood cortisol levels following Dexamethasone Suppression Tests in older individuals compared to younger individuals with bipolar disorder. Older people with bipolar disorder have presumably experienced the chronic stress associated with the disorder for a prolonged time, and in the long term this may lead to down-regulation of the HPA axis. Similarly, Pochigaeva et al. (2017) found higher plasma cortisol concentrations in patients with major depression disorder, but lower hair cortisol values, which suggests that this disorder is also associated with increased downregulation of the HPA axis. In our study, individuals who behaved more emotionally as infants during BioBehavioral Assessment may have continued to show that same behavioral pattern in response to other stressors they encountered throughout their lives, including the new group formation. The experience of chronic activation of the HPA axis could eventually lead to down-regulation of this system. While we do not have behavioral or physiological data on our subjects between the time of the BBA assessment and the group formation, we contrasted animals that were above versus below the median on Day 1 Emotionality on the change in plasma cortisol from our first sample (taken two hours after arrival for BBA testing; data not presented here) with the second sample (described herein as the Afternoon Response), taken five hours later. Consistent with the notion that these two sets of animals respond differently to stressors, we found that animals high on Day 1 Emotionality showed a 9 ug/dl increase in plasma cortisol concentrations over this five hour period, while the low Day 1 Emotionality animals showed a 5 ug/dl decrease in cortisol concentrations. Although the result was not significant (p=0.182, data not shown), it is in the direction expected if high Day 1 Emotionality individuals are showing poorer coping in the BioBehavioral Assessment situation.
We propose, then, that high Day 2 Activity indexes adaptability, a trait that confers benefits in navigating social encounters that might otherwise result in severe or repeated trauma, while high Day 1 Emotionality does not. The lower cortisol concentrations for the high Day 2 Activity animals reflect, we believe, adaptive coping, while the lower cortisol concentrations for the high Day 1 Emotionality animals reflect poorer coping, results that are consistent with the BioBehavioral Assessment program’s previous research on Emotionality and Activity, described above, as well as the post-hoc analysis on trauma presented here. We consider this interpretation provisional at this point, requiring more prospective study, but we suggest that at the very least, these results add to the literature suggesting that caution is required in interpreting differences in hair cortisol values among individuals; in particular, the idea that lower values of hair cortisol are always ‘better’ may not be correct.
Afternoon Response and Dexamethasone Suppression Test
Individuals with higher blood cortisol values following Dexamethasone Suppression Tests during BioBehavioral Assessment in infancy also had higher hair cortisol values following group formation later in life, as predicted. This suggests some stability in inter-individual differences in HPA axis regulation, such that infants with strong down-regulating mechanisms become adults with strong down-regulating mechanisms. This offers support to the idea that BioBehavioral Assessment conducted once in infancy can characterize stable traits of the individual. We also found that, contrary to predictions, individuals with higher blood cortisol concentrations in their Afternoon Response to BioBehavioral Assessment went on to exhibit lower hair cortisol concentrations later in life after the new group formation. Ultimately, the increase in cortisol following a stressor is associated with an adaptive response that presumably helps the individual deal with the stressor (Sapolsky et al., 2000). If the stressor is dealt with more effectively, this could decrease the need for future stress responses, which might ultimately decrease long-term HPA axis activity. However, it might also be that these individuals’ HPA axes have become down-regulated due to chronic high cortisol production earlier in life, similar to one of our proposed explanations for lower hair cortisol concentrations in highly emotional individuals. For example, a greater cortisol response to BioBehavioral Assessment might indicate poorer coping with stressors more generally, which could eventually lead to down-regulation of the HPA axis.
Nervous Temperament and Preference for Novelty
We did not find evidence that Nervous temperament was associated with changes in hair cortisol concentrations following the new group formation. We initially hypothesized that more nervous individuals would have higher hair cortisol levels after the group formation, based on previous work showing highly nervous individuals exhibit more negative emotional responses to a Human Intruder Test during BioBehavioral Assessment (Capitanio et al., 2011). Nervous temperament might therefore be compared to Emotionality, in that both metrics are correlated with some measure of negative emotional responsiveness exhibited during different portions of BioBehavioral Assessment. It is surprising, then, that Nervous temperament is not associated with changes in cortisol after group formation, while Emotionality is. However, we note that Nervous temperament and Emotionality are not correlated in our sample, meaning these metrics index different characteristics. One key difference between these metrics is that the negative emotional responses that Capitanio et al. (2011) reported in highly nervous individuals occur during one portion of testing, in response to a specific threatening stimulus: a human intruder who moves close to the animal and maintains eye contact with it. In contrast, the negative emotional responses encompassed in Emotionality scores are exhibited outside of the Human Intruder Test, in the absence of this direct threat. Highly emotional individuals might therefore have a lower threshold for exhibiting these behaviors than highly nervous individuals do. If emotionality is indeed associated with HPA axis downregulation, this might explain why highly nervous individuals do not suffer the same consequences: they do not exhibit negative emotional reactions in as wide a range of circumstances as highly emotional individuals do.
We also did not find that Preference for Novelty was associated with changes in hair cortisol values following group formation. Previous work has shown that high Preference for Novelty was associated with greater sociability later in life (Sclafani et al., 2016). We hypothesized that this tendency to be more sociable would help individuals adapt to the new social setting following the new group formation, leading to lower hair cortisol concentrations. Differences in individuals’ sociability may not affect how stressful a new group formation is, or the effects may be too small to detect in our current sample.
Summary and Future Directions
We have demonstrated that inter-individual differences in temperament measured in infancy predict physiological responses to new group formation years later, although some of the associations of these measures with hair cortisol were not in the predicted direction. There are a number of possible explanations for the relationships we have found between temperament characteristics and hair cortisol values following new group formation. Based on previous work on Emotionality and Activity, we favor the explanation that there are two ways in which individuals can reach the outcome of low 9-month Hair Cortisol: either through chronic stress leading to down-regulation of the HPA axis (higher Day 1 Emotionality) or through adaptive coping (as indexed by higher Day 2 Activity). This interpretation is most consistent with other lines of evidence indicating that in captivity high Day 1 Emotionality indexes poor coping with stressors and that high Day 2 Activity indexes successful coping with stressors. However, due to our small sample size and the lack of data on later life HPA axis regulation, this is necessarily a speculative conclusion. It is also possible that both high Day 1 Emotionality and high Day 2 Activity lead to successful coping, or that both lead to HPA axis dysregulation. Additional research is required to distinguish between low cortisol due to successful coping and low cortisol due to HPA axis dysregulation.
Our results lead us to several specific avenues for future study. First, the most critical follow-up is to assess changes in HPA axis function over the course of a period of social instability, such as that following a new group formation. It remains to be determined what the exact mechanism is by which greater early-life measures of Day 1 Emotionality and Day 2 Activity produce lower hair cortisol values. HPA axis function could be assessed repeatedly via plasma cortisol responses to Dexamethasone Suppression Tests before and after a new group formation. If our interpretation is correct, individuals with high Day 1 Emotionality will have increased sensitivity to negative feedback inhibition of the HPA axis (as, for example, in rhesus macaques in socially unstable housing: Capitanio et al., 1998), while individuals with high Day 2 Activity will not. Similarly, longitudinal studies of individuals’ cortisol production and HPA axis regulation could compare high-Afternoon Response and low-Afternoon Response groups to see if the former experiences chronically high cortisol levels, eventually leading to down-regulation of the HPA axis. Incorporating data from later-life health outcomes besides HPA activity could also strengthen the importance of these findings by illustrating a cascading effect of temperament on HPA activity, which in turn might influence disease risk, reproductive function, or other evolutionarily significant parameters.
Second, future work on social instability should examine behavioral and social correlates of HPA activity during periods of social instability. Behavioral data demonstrating continued differences in Activity and Emotionality following captive group formations would support our interpretation that these early-life measures reflect stable individual differences in responding to novel stressors. Behavioral data during the period immediately following a new group formation would also clarify how certain individuals avoided severe or recurrent physical trauma, while others did not. Rank is an important organizer of an individual’s social experience, but we could not examine rank here due to limitations in sample size. Future studies could expand on our findings by examining whether rank, HPA activity, and temperament are correlated. If these measures are related, behavioral data will be crucial in determining the mechanism by which they influence one another. For example, individuals with particular temperament traits might also achieve higher rank, which might in turn affect HPA activity. Behavioral data might also help explain why Nervous temperament and Preference for Novelty were not associated with changes in hair cortisol concentrations following group formation. For example, such data could tell us whether highly Emotional individuals do indeed respond emotionally to a broader range of circumstances than highly Nervous individuals do.
Finally, we examined only one group formation here. This group formation combined several fairly large subgroups within which individuals were familiar with one another, along with some indoor-housed individuals who were unfamiliar with their new groupmates. Future studies could examine captive group formations involving subgroups of varying sizes, for example consisting of smaller groups closer to the size seen in wild group fusion events. Larger studies comparing multiple new group formations could also examine whether variation in group composition (e.g., age, sex, temperament) affects HPA activity. For example, the temperaments of others in the new group might affect which temperaments are most successful in coping with social instability following a new group formation.
Supplementary Material
Acknowledgments
Hair cortisol work was supported by two research grants to JBL from the Department of Anthropology at UC Davis and by a UC Davis Faculty Research Grant to LAI. Hair sample collections were conducted under IACUC protocol #18090. We thank the CNPRC staff who assisted in hair sample collection, those who collect and maintain colony records used in this study, and B. Beisner, A. Cameron, D. Hannibal, J. Vandeleest, and H. Martin for helpful discussion. We also thank E. Van Cleave, M. Tran, R. Montez, E. Rivera, L. Milgrom, H. Martyn, J. Hook, and M. Gelatos for help with hair processing, L. Calonder and L. Del Rosso for collecting BioBehavioral Assessment data, and M. Grote for assistance with statistical analysis. Several reviewers offered detailed and constructive comments that improved the manuscript considerably and we thank them all. BioBehavioral Assessment work was supported by NIH grant R24OD010962 (Capitanio). The lab in which hair cortisol analysis was performed is supported by NIH R01HD068335 (McCowan). The CNPRC is funded by the base grant P51OD011107 (Lewin). The authors declare no conflicts of interest.
References
- Alberts SC, Sapolsky RM, & Altmann J (1992). Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Hormones and Behavior 26(2), 167–178. DOI: 10.1016/0018-506X(92)90040-3 [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Weladji RB, & Paré P (2016). Changes in dominance hierarchy of captive female Japaneses macaques as a consequence of merging two previously established groups. Zoo Biology 35(6), 505–512. DOI: 10.1002/zoo.21322 [DOI] [PubMed] [Google Scholar]
- Beaulieu M, Sylvère M, Willaume E, Kappeler PM, & Charpentier MJE (2014). The oxidative cost of unstable social dominance. Journal of Experimental Biology 217(15), 2629–2632. DOI: 10.1242/jeb.104851 [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ (2017). The next step for stress research in primates: to identify relationships between glucocorticoid secretion and fitness. Hormones and Behavior 91(May 2017), 68–83. DOI: 10.1016/j.yhbeh.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron AN, & McCowan B (2011). Detecting instability in animal social networks: genetic fragmentation is associated with social instability in rhesus macaques. PLoS ONE 6(1), e16365 DOI: 10.1371/journal.pone.0016365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcorne F, Fuentes A, Wandia IN, Beudels-Jamar RC, & Huynen MC (2015). Changes in activity patterns and intergroup relationships after a significant mortality event in commensal long-tailed macaques (Macaca fascicularis) in Bali, Indonesia. International Journal of Primatology 36(3), 548–566. DOI: 10.1007/s10764-015-9841-5 [DOI] [Google Scholar]
- Capitanio JP (1999). Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. American Journal of Primatology 47(4), 299–320. DOI: 10.1002/(SICI)1098–2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Capitanio JP (2011). Individual differences in emotionality: social temperament and health. American Journal of Primatology 73(6), 507–515. DOI: 10.1002/ajp.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP (2017). Variation in BioBehavioral Organization In Schapiro S (Ed.), Handbook of Primate Behavioral Management (pp. 55–73). Boca Raton, FL: CRC Press. [Google Scholar]
- Capitanio JP, Abel K, Mendoza SP, Blozis SA, McChesney MB, Cole SW, & Mason WA (2008). Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain, Behavior, and Immunity 22(5), 676–689. DOI: 10.1016/j.bbi.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Blozis SA, Snarr J, Steward A, McCowan BJ (2017). Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. American Journal of Primatology 79(1), e22464 DOI: 10.1002/ajp.22464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, & Cole SW (2015). Social instability and immunity in rhesus monkeys: the role of the sympathetic nervous system. Philosophical Transactions B 370, 20140104 DOI: 10.1098/rstb.2014.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso L, & Roberts JA (2006). Nursery rearing and biobehavioral organization In: Sackett GP, Ruppentahal GC, Elias K, editors. Nursery rearing of nonhuman primates in the twenty-first century (pp. 191–214). New York, NY: Springer Science. [Google Scholar]
- Capitanio JP, Mendoza SP, & Cole SW (2011). Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol’s influence on trafficking. Brain, Behavior, and Immunity 25(1), 151–159. DOI: 10.1016/j.bbi.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, & Mason WA (1998). Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proceedings of the National Academy of Sciences of the United States of America 95(8), 4714–4719. DOI: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, & Maninger N (2005). Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta). Developmental Psychobiology 46(4), 318–330. DOI: 10.1002/dev.20067 [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, & Meyer JS (2006). Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology, 147, 255–261. 10.1016/j.ygcen.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Fourie NH, & Bernstein RM (2011). Quantification of cortisol in wild and captive nonhuman primate hair: methodological considerations and biological validation. American Journal of Physical Anthropology 52(S), 137. [Google Scholar]
- Freeman HD, & Gosling SD (2010). Personality in nonhuman primates: a review and evaluation of past research. American Journal of Primatology 72(8), 653–671. DOI: 10.1002/ajp.20833 [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, & Capitanio JP (2009). Iron deficiency anemia and affective response in rhesus monkey infants. Developmental Psychobiology 51(1), 47–59. DOI: 10.1002/dev.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygax L, Harley N, and Kummer H (1997). A matrilineal overthrow with destructive aggression in Macaca fascicularis. Primates 38(2), 149–158. DOI: 10.1007/BF02382005 [DOI] [Google Scholar]
- Hamel AF, Lutz CK, Coleman K, Worlein JM, Peterson EJ, Rosenberg KL, Novak MA, Meyer JS (2017). American Journal of Primatology 79:e22526 DOI: 10.1002/ajp.22526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, McCowan B, Jiang J, & Capitanio JP (2014). Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Frontiers in Behavioral Neuroscience 8, 1–8. DOI: 10.3389/fnbeh.2014.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, & Capitanio JP (2015). Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology 26(1), 269–281. DOI: 10.1093/beheco/aru186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, & Blokhuis HJ (1999). Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews 23(7), 925–935. DOI: 10.1016/S0149-7634(99)00026-3 [DOI] [PubMed] [Google Scholar]
- Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF (2017). Hyper- and hypocortisolism in bipolar disorder – a beneficial influence of lithium on the HPA-axis? Journal of Affective Disorders 213(15 April 2017), 161–167. DOI: 10.1016/j.jad.2017.02.026 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Wingfield JC (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior 43(1), 2–15. DOI: 10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- Melhem NM, Munroe S, Marsland A, Gray K, Brent D, Porta G, Douaihy A, Laudenslager ML, DePietro F, Diler R, Driscoll H, Gopalan P (2017). Blunted HPA axis activity prior to suicide attempt and increased inflammation in attempters. Psychoneuroendocrinology 77(March 2017), 284–294. DOI: 10.1016/j.psyneuen.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, & Novak MA (2012). Minireview: Hair cortisol: A novel biomarker of Hypothalamic-Pituitary-Adrenocortical activity. Endocrinology 153(9), 4120–4127. doi: 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, and R Core Team (2018). nlme: Linear and Nonlinear Mixed Effects Models R package version 3.1-131.1. [Google Scholar]
- Pochigaeva K, Druzhkova T, Yakovlev A, Onufriev M, Grishkina M, Chepelev A, … Gulyaeva N (2017). Hair cortisol as a marker of hypothalamic-pituitary-adrenal axis activity in female patients with major depressive disorder. Metabolic Brain Disease 32(2), 577–583. DOI: 10.1007/s11011-017-9952-0 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, & Dingemanse NJ (2007). Integrating animal temperament within ecology and evolution. Biological Reviews 82(2), 291–318. DOI: 10.1111/j.1469-185X.2007.00010.x [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE (2009). The reactive scope model – A new model integrating homeostasis, allostasis, and stress. Hormones and Behavior 55(3), 375–389. DOI: 10.1016/j.yhbeh.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Rommeck I, Capitanio JP, Strand SC, & McCowan B 2011. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta). American Journal of Primatology 73(7), 692–701. DOI: 10.1002/ajp.20953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels A, Silk JB, & Altmann J (1987). Continuity and change in dominance relations among female baboons. Animal Behaviour 35(3), 785–793. DOI: 10.1016/S0003-3472(87)80115-X [DOI] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21(1), 55–89. DOI: 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Sclafani V, Del Rosso LA, Seil SK, Calonder LA, Madrid JE, Bone KJ, … Parker KJ (2016). Early predictors of impaired social functioning in male rhesus macaques (Macaca mulatta). PLoS ONE 11(10): e0165401 DOI: 10.1371/journal.pone.0165401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB (2009). Nepotistic cooperation in non-human primate groups. Philosophical Transactions of the Royal Society B 364(1533), 3243–3254. DOI: 10.1098/rstb.2009.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T, la Fleur S, & Schulz PE (1997). Neuroendocrine correlates of emotional reactivity and coping in male rats from the Roman high (RHA/Verh)- and low (RLA/verh) avoidance lines. Behavior Genetics 27(6), 503–512. DOI: 10.1023/A:1021448713665 [DOI] [PubMed] [Google Scholar]
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schönfeld S, Hoyer J, & Stalder T (2013). Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biological Psychiatry 74(9), 639–646. DOI: 10.1016/j.biopsych.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Suomi SJ (1997). Early determinants of behavior: evidence from primate studies. British Medical Bulletin 53(1), 170–184. DOI: 10.1093/oxfordjournals.bmb.a011598 [DOI] [PubMed] [Google Scholar]
- Wechsler B (1995). Coping and coping strategies: a behavioural view. Applied Animal Behaviour Sciences 43(2), 123–134. DOI: 10.1016/0168-1591(95)00557-9 [DOI] [Google Scholar]
- Weinstein TAR, & Capitanio JP (2008). Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behaviour 76(2), 455–465. DOI: 10.1016/j.anbehav.2008.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME (2016). An introduction to the female macaque model of social subordination stress In Shively CA and Wilson ME (Eds.), Social Inequalities in Health in Nonhuman Primates (pp. 9–24). New York, NY: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.