Retroviruses expressing enhanced green fluorescent protein (eGFP) or other similar reporter genes are the current gold standard for labeling newly born cells in the postnatal brain. Previous methods had notable disadvantages. Bromodeoxyuridine (BrdU) birthdating methodologies, which involve incorporation of the thymidine analog into cells synthesizing DNA, can spuriously label cells undergoing aborted DNA synthesis after insult (Kuan et al., 2004). There are also technical difficulties associated with demonstrating colocalization of BrdU with neuronal markers (Rakic, 2002). However, retroviruses seemed a good solution because they specifically transduce dividing cells as a result of a breakdown of the nuclear envelope during the M phase of the cell cycle. They carry with them the added benefit of allowing visualization of the entire cell and electrophysiological recording. Based on the use of retroviruses in confirming integration of newborn neurons into the dentate gyrus and olfactory bulb, Ackman et al. (2006), in a recent issue of The Journal of Neuroscience, attempted to label new cortical neurons. They report unexpected but nonetheless exciting results.
Labeling cells with retrovirus is rather inefficient because of the limits of viral titer, the relatively small population of dividing cells in the postnatal brain, and limited spread of virus from the injection site. To counter this limitation, Ackman et al. (2006) injected 134 postnatal rats, the |P′brute force|P' approach. Of these brains, they found 642 eGFP-labeled neurons [Ackman et al. (2006), their Table 1 (http://www.jneurosci.org/cgi/content/full/26/44/11413/T1)]. Ostensibly, this would have been confirmation of neuronogenesis in the postnatal mammalian neocortex. However, labeled pyramidal neurons with mature morphology were only found 2 d after injection yet were not found in animals killed 3 weeks later [Ackman et al. (2006), their Table 1 (http://www.jneurosci.org/cgi/content/full/26/44/11413/T1), Fig. 1 (http://www.jneurosci.org/cgi/content/full/26/44/11413/F1)]. Because of the fact that it takes weeks for a newborn pyramidal cell to migrate and mature in the postnatal cortex, and because the eGFP labeled neurons could not be labeled with BrdU [Ackman et al. (2006), their Fig. 2 (http://www.jneurosci.org/cgi/content/full/26/44/11413/F2)], the results indicated that the labeled neurons were not newborn. Keen inspection of the eGFP-labeled pyramidal cells indicated the presence of a second cell body fused to the apical dendrites [Ackman et al. (2006), their Fig. 3 (http://www.jneurosci.org/cgi/content/full/26/44/11413/F3)]. Because of the small number of labeled neurons in vivo, the authors also used an in vitro system. In cultures of cortical neurons, addition of eGFP retrovirus led to the labeling of mature neurons within 2 d, which then persisted for 14 d [Ackman et al. (2006), their Fig. 4 (http://www.jneurosci.org/cgi/content/full/26/44/11413/F4)]. Immunostaining of the secondary cell bodies fused to eGFP+ neurons indicated that they were microglia [Ackman et al. (2006), their Fig. 5 (http://www.jneurosci.org/cgi/content/full/26/44/11413/F5)].
Thus, the authors hypothesized that dividing microglial cells incorporated the retrovirus and subsequently fused to mature neuronal dendrites, filling the cytoplasm with eGFP. Several elegant experiments confirmed this hypothesis. First, blocking cell proliferation in vitro with cytosine arabinoside prevented eGFP incorporation, indicating that the retrovirus was incorporated only into dividing cells [Ackman et al. (2006), their supplementary Fig. 3 (http://www.jneurosci.org/content/vol26/issue44/images/data/11413/DC1/supplfig3.gif)]. Second, the microglia that fused to the neurons incorporated BrdU while the neuron did not [Ackman et al. (2006), their supplemental Fig. 6 (http://www.jneurosci.org/content/vol26/issue44/images/data/11413/DC1/supplfig6.gif)]. Third, activation of microglia with lipopolysaccharide (LPS) increased fusion in vitro >10-fold [Ackman et al. (2006), their Fig. 7E (http://www.jneurosci.org/cgi/content/full/26/44/11413/F7)]. Although virus in the culture was necessary for fusion, fused neuronal–microglial cells did not necessarily need to be transduced because fused GFP− pairs were found [Ackman et al. (2006), their supplemental Fig. 5 (http://www.jneurosci.org/content/vol26/issue44/images/data/11413/DC1/supplfig5.gif)]. In a few GFP+ neurons, distally fused microglia were not found. Instead, the presence of two distinct nuclei was noted in the soma [Ackman et al. (2006), their supplemental Fig. 4 (http://www.jneurosci.org/content/vol26/issue44/images/data/11413/DC1/supplfig4.gif)]. Together, these results clearly demonstrate that viral presence causes the fusion of activated microglia to the dendrites of mature pyramidal cells in the postnatal cortex.
Overall, the authors set out in search of new neurons in the cortex and instead stumbled on an intriguing phenomenon of neuronal–microglial fusion. The comprehensiveness of their approach of viral injections argues against large-scale addition of neurons to the neocortex postnatally. This is in agreement with the majority of studies in rodents, monkeys, and humans. This study also indicates that the use of retroviral labeling as a marker of newborn cells has its caveats, particularly in situations in which there may be increased inflammation and microglial activation. Furthermore, these findings along with previous reports of fusion, suggest the need for increased scrutiny in transplantation or transdifferentiation experiments, where neuronal fusion has been noted. (Terada et al., 2002; Ying et al., 2002; Alvarez-Dolado et al., 2003). However, in the previous reports, the fusogenic cell could not be determined, despite the presence of binucleate or fused nuclei. This report indicates that the fusion event may start at the distal dendrites as a potential precursor or alternative to somal fusion. Such a rare distal fusion event might not be readily visible in thin sections, especially if the survival time is longer than 2 weeks.
Several questions arise from this study. Is there a functional significance for the fusion of microglia to neurons in reactive conditions, or is it just an epiphenomenon caused by the retrovirus? Why were labeled neurons not seen in animals injected at later ages? Could fusion be a precursor to cell death or a form of plasticity? The fusion events observed were rare and eGFP labeling was transient. It might be interesting to transduce primary microglia ex vivo (Nakajima et al., 1992) with the retrovirus (perhaps stimulated by LPS), followed by stereotaxic transplantation into the cortex or by the addition of these cells to primary cortical neuron culture or slice culture. This might allow a comprehensive observation of the process by exponentially increasing the number of observable fusion events. Also, what is the molecular mechanism of this fusion and why is fusion specific to the apical dendrite? The authors speculate that the vesicular stomatitis virus glycoprotein G (VSV-G) envelope protein may mediate this process as it contains a fusogenic domain and is known to cause membrane fusion between neighboring cells in vitro. Additional studies using an alternate VSV-G pseudotyped eGFP-expressing retrovirus or the same gapEGFPm4 virus in the absence of the VSV-G envelope protein would answer this question. If VSV-G is the culprit, clinical uses of pantrophic VSV-G-containing or VS viruses for gene therapy (Johnson et al., 2007) must take care to ensure that fusion to postmitotic off-target cells is not a significant issue.
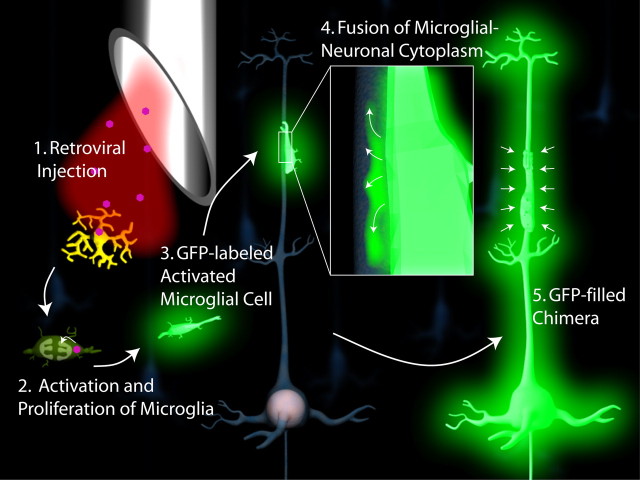
Figure 1.
Fusion of microglial cell to mature neuron stimulated by injection of retrovirus. 1, Stereotaxic injection of retrovirus into the forebrain infects microglia and stimulates their activation (2). 3, Activated microglial cell migrates and recognizes apical dendrite of a mature cortical pyramidal cell where it fuses, filling the cell with GFP (4). 5, Entire cytoplasm of the neuron is filled with GFP.
Ackman et al. (2006) have discovered a novel fusogenic interaction. As they discuss, with proper insight into the precise mechanism of neuronal–microglial fusion, this phenomenon might be co-opted as a novel gene delivery vector which bypasses the blood–brain barrier or used as a means of nuclear reprogramming through fusion.
Footnotes
Editor's Note: These short reviews of a recent paper in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to mimic the journal clubs that exist in your own departments or institutions. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
References
- Ackman JB, Siddiqi F, Walikonis RS, LoTurco JJ. Fusion of microglia with pyramidal neurons after retroviral infection. J Neurosci. 2006;26:11413–11422. doi: 10.1523/JNEUROSCI.3340-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K, Lee M, Reilly PA, Clarke DK, Hendry RM, Udem SA. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in nonhuman primates. Virology. 2007 doi: 10.1016/j.virol.2006.10.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Shimojo M, Hamanoue M, Ishiura S, Sugita H, Kohsaka S. Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem. 1992;58:1401–1408. doi: 10.1111/j.1471-4159.1992.tb11356.x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primates. Prog Brain Res. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]