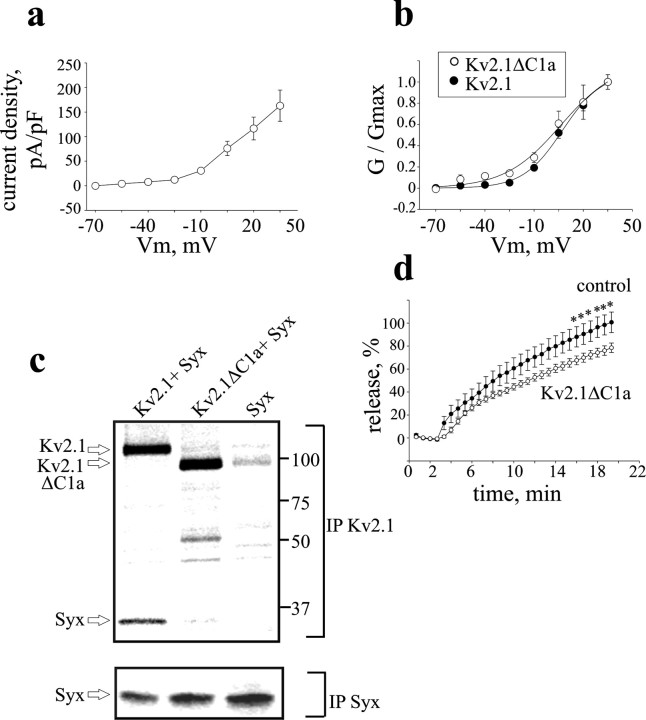
Figure 5.
A Kv2.1 mutant with impaired syntaxin-binding capacity does not enhance release. a, Averaged current densitiy–voltage relationships in PC12 cells transfected with the mutant channel (Kv2.1 ΔC1a; n = 6), derived as in Figure 2b. Note that the current densities of the mutant channel are similar to those of the wild-type channel (compare with Fig. 2b). b, Normalized conductance (G/Gmax)–voltage relationship of Kv2.1 ΔC1a superimposed on that of Kv2.1 (from Fig. 2c). c, Physical interaction of the Kv2.1ΔC1a channel with syntaxin in Xenopus oocytes is impaired. Digitized PhosphorImager scans of SDS-PAGE analysis of [35S]Met/Cys-labeled Kv2.1, Kv2.1ΔC1a, and syntaxin proteins coprecipitated by either anti-Kv2.1 antibody (IP Kv2.1) or anti-syntaxin antibody (IP Syx) from oocytes that were injected with Kv2.1 or Kv2.1ΔC1a together with syntaxin (+Syx), or injected with syntaxin alone (Syx) are shown. Arrows indicate the relevant proteins. The scans show that the amount of syntaxin coprecipitated with mutant channel (see IP Kv2.1) is significantly smaller than that coprecipitated with the same amount wild-type channel, although expression of syntaxin in oocytes expressing the mutant channel was not smaller than that of the wild type (see IP Syx). The results shown are from one of four independent experiments. d, Kv2.1ΔC1a overexpression does not enhance neuropeptide release induced by depolarization with high external KCl concentration. Cells were transfected with the mutant (Kv2.1ΔC1a) or with empty vector (control) (n = 12 and 10, respectively). *p < 0.05.