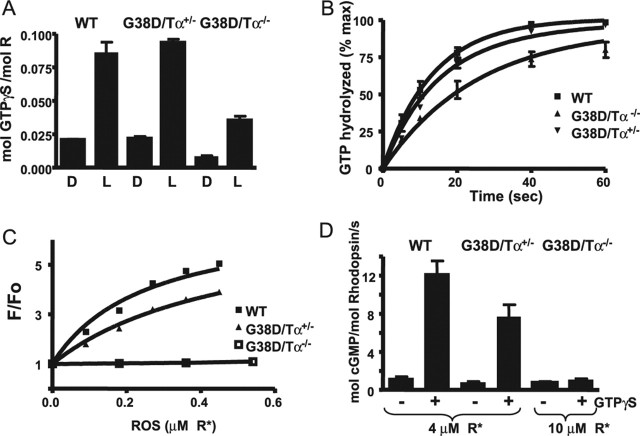
Figure 4.
A, Light-dependent binding of GTPγS to transducin in the ROSs from control and transgenic mice. ROSs isolated from dark (D)-adapted mice (12 μl) containing 10 μm rhodopsin were mixed with 6 μl of 10 μm [35S]GTPγS under infrared illumination. After a 10 s incubation, the amount of bound GTPγS was determined in a 7 μl aliquot by the nitrocellulose filter binding assay. The rest of the sample was bleached to determine the GTPγS binding in the light (L). Error bars indicate SE (n = 3). B, Transducin GTPase activities in the ROSs from control and transgenic mice. The single-turnover GTPase reactions were started by mixing bleached ROSs (17 μl) containing 10 μm rhodopsin with 17 μl of 0.1 μm [γ-32P]GTP. The time course of 32Pi formation was determined using the activated charcoal procedure after the reactions were stopped with perchloric acid. The GTPase rate constants from one-phase exponential fits for the control and G38D/Tα−/− ROSs were 0.076 ± 0.005 and 0.039 ± 0.003 s−1 (mean ± SE; n = 3). The data for G38D/Tα+/− ROS were analyzed with two-phase exponential fit (Y1max fixed at 75% and k1 fixed at 0.076 s−1) yielding a k2 of 0.032 s−1. max, Maximium. C, Fluorescence PγBC binding assay. The relative fluorescence change (F/Fo) of PγBC (10 nm; excitation, 445 nm; emission, 495 nm) was determined after the addition of increasing concentrations of the ROSs from control, G38D/Tα+/−, and G38D/Tα−/− mice in the presence of 1 μm GTPγS. The results from one of three similar experiments are shown. D, PDE activation assay. Bleached ROSs from control and G38D/Tα+/− mice (4 μm R*) and from G38D/Tα−/− mice (10 μm R*) were incubated with 3 μm cGMP for 3 min to stabilize the PDE6 basal activity. The reactions were started by the addition of 2 mm [3H]cGMP with or without 2 μm GTPγS. cGMP hydrolysis was terminated after 10 s by heating the samples for 2 min at 100°C, and the amount of hydrolyzed cGMP was measured as described in Materials and Methods. Error bars indicate SE (n = 3). WT, Wild type; mol, molecular.