Figure 3.
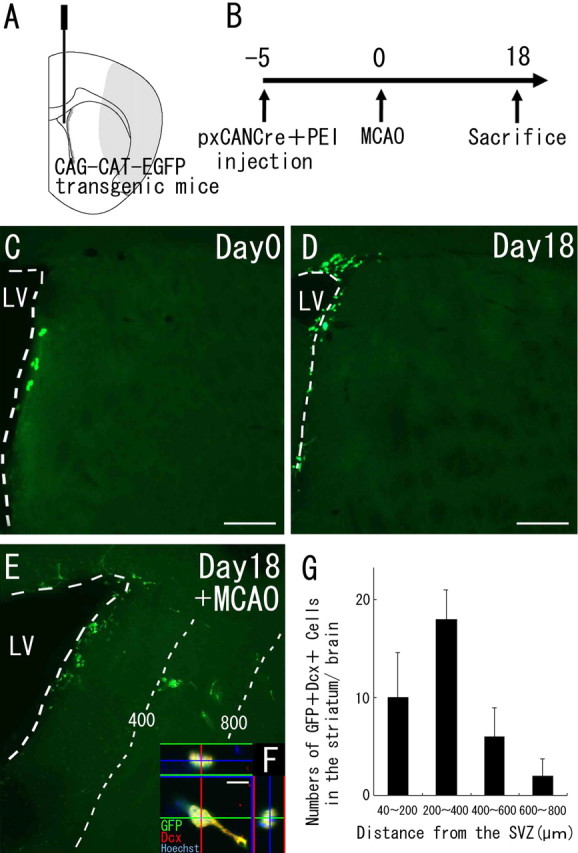
Neuroblasts migrate from the SVZ into the striatum after cerebral ischemia. A, Diagram showing the injection site. The Cre-encoding pxCANCre plasmid was injected into the lateral ventricles of CAG-CAT-EGFP transgenic mice, which carry the floxed EGFP gene under control of the CAG promoter. B, pxCANCre plasmid plus PEI was injected into the lateral ventricle 5 d before MCAO, and the animals were killed 18 d after MCAO. C, D, In the normal brain (n = 4 at each time point), GFP-positive cells (green) are observed only in the SVZ at 5 and 23 d after the injection (corresponding to days 0 and 18, respectively, in B). Scale bars, 200 μm. E, In the ischemic brain (n = 4), many SVZ-derived GFP-positive cells (green) are observed in the peri-infarct striatum 23 d after the injection (corresponding to day 18 in B). The numbers and thin dotted lines indicate distance (in micrometers) from the SVZ. F, High-power confocal image of a GFP/Dcx-expressing cell exhibiting morphology typical of migrating neuroblasts. Thirty-seven percent of the GFP-positive cells (n = 45) in the striatum expressed Dcx. Scale bar, 10 μm. G, Numbers of GFP/Dcx double-positive cells in four regions at different distances from the SVZ 18 d after MCAO. This distribution is very similar to those of GFP-positive cells (Fig. 1D). No SVZ-derived GFP/Dcx double-immunopositive cells were observed in the striatum of the non-ischemic brain.
