Figure 10.
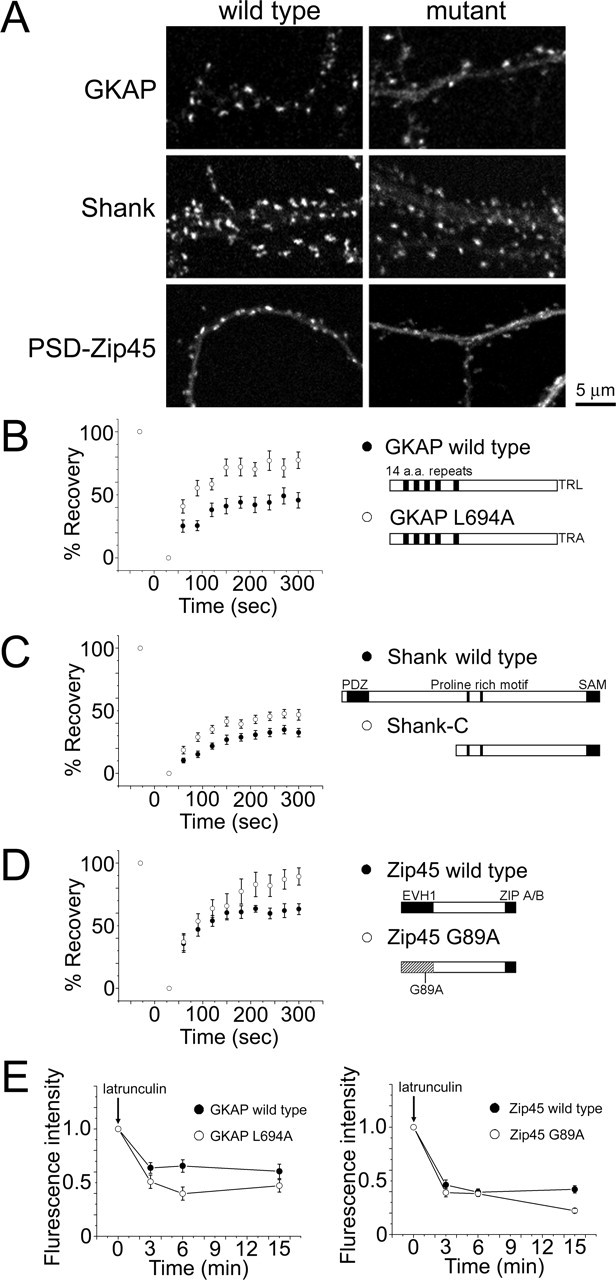
Dynamic properties of GKAP, Shank, and PSD-Zip45 molecules containing mutations that block interaction with other scaffolding proteins. A, Distribution of mutated forms of GKAP, Shank, and PSD-Zip45 tagged with EGFP within dendrites. The extent of synaptic clustering is reduced in all mutants compared with the wild-type proteins. Scale bar, 5 μm. B–D, FRAP kinetics of GKAP L694A (B), Shank-C (C), and PSD-Zip45 G89A (D). Marked enhancement of FRAP kinetics was observed in GKAP L694A and PSD-Zip45 G89A. E, Enhancement of latrunculin A-dependent loss of GKAP and PSD-Zip45 by introduction of mutations disrupting their interactions with other scaffolding proteins. Error bars indicate SEM.
