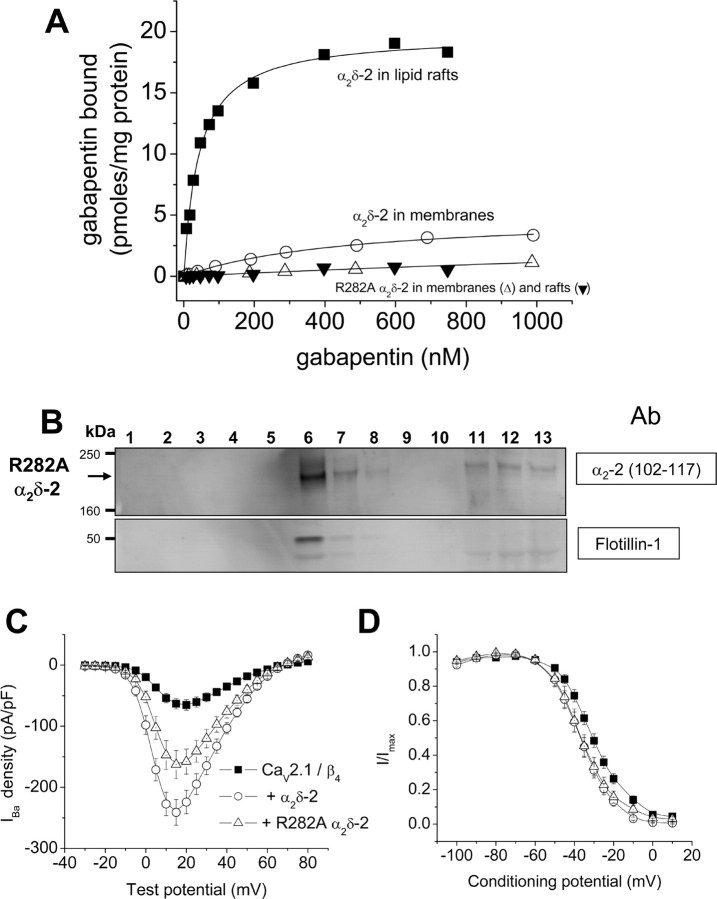
Figure 9.
Gabapentin binding to α2δ-2(HA) cell line is enhanced in cholesterol-enriched microdomains and prevented by R282A mutation. A, Examples of experiments to determine the concentration of [3H]gabapentin binding sites and affinity for [3H]gabapentin of a membrane fraction (○; KD of 468 nm; representative example from n = 3) and cholesterol-rich microdomains (■; KD of 42.6 nm; representative example from n = 4) derived from the α2δ-2(HA) cell line. Also included on this graph are examples of the lack of binding of [3H]gabapentin to membranes (Δ; representative example from n = 5) or lipid rafts (▾; representative example from n = 2) prepared from Cos-7 cells transiently expressing the R282A α2δ-2 mutant. B, Western blot analysis of the distribution of proteins found in fractions from a typical discontinuous sucrose gradient, derived from transient expression of R282A α2δ-2. Fractions 1–13 from the sucrose gradient (run under disulfide-bond-reducing conditions) were probed for α2δ-2-derived products with Ab α2-2(102–117) (top). The bottom shows the location of the cholesterol-rich microdomain marker protein flotillin-1. C, Comparison of the effect of expression of α2δ-2 and R282A α2δ-2 on currents through Cav2.1/β4 channels in tsA-201 cells, using Ba2+ as charge carrier. Current density–voltage relationships (mean ± SEM) are shown for the three experimental conditions: ■, Cav2.1/β4 (n = 14); ○, with α2δ-2 (n = 14); and Δ, with R282A α2δ-2 (n = 18). D, Comparison of the effect of expression of α2δ-2 and R282A α2δ-2 on the steady-state inactivation of currents through Cav2.1/β4 channels in tsA-201 cells. Conditioning prepulses of 5 s to the potentials shown were given before a constant test pulse to +10 mV. The normalized data (mean ± SEM) are shown for the three experimental conditions: ■, Cav2.1/β4 (n = 11); ○, with α2δ-2 (n = 11); and Δ, with R282A α2δ-2 (n = 9). The V50 values are −30.5 ± 0.4, −36.2 ± 0.5, and −36.5 ± 0.4 mV, and the k values are 8.4 ± 0.4, 8.0 ± 0.4, and 8.1 ± 0.4 mV, respectively.