Figure 3.
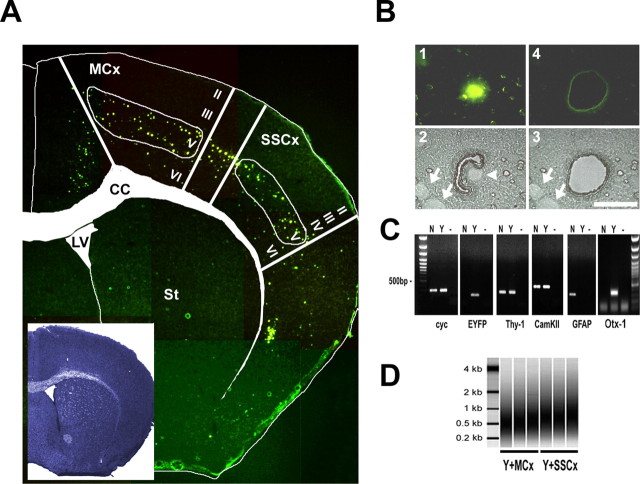
Fluorescence-directed laser microdissection enables the isolation of neuronal cell types with high precision. A, Fluorescent micrographs taken from an 8 μm TYNC brain cryosection mounted on a POL foil slide at the level of the striatum (St) (bregma, 1 mm) were manually assembled to an overview picture. The inset shows an adjacent section stained with thionin. For better orientation, the boundaries of the corpus callosum (CC) and of the lateral ventricle (LV) were marked in the thionin section, and the label was transferred to the fluorescent picture. In the cortex, at the level of the primary motor and somatosensory areas (MCx and SSCx), mainly cells of the deeper layers are EYFP positive (layer V and few cells in layer VI). In the upper layers II, III, and IV, a low number of dispersed cells were EYFP positive, usually with a weaker EYFP signal intensity. Samples isolated with laser microdissection were chosen from the primary motor and the somatosensory regions. B, Fluorescence-directed laser microdissection of a single YFP-positive cortical neuron. 1, In the fluorescent mode, a single cell was located and marked by an appropriate painting tool (Leica LDM software); only strong EYFP-positive cells in the plane of section were chosen for isolation. The laser-cutting line was drawn around the nucleus at a distance of half the diameter of the nucleus at the most. 2, The microscope was subsequently switched to bright-field mode; here, the corresponding nucleus is visible as a bright round structure (arrowhead). Cells with other nuclei in close proximity were not isolated. Depicted are two nuclei (arrows) at a distance where contamination was considered to be low. Laser cutting follows a manually or automatically drawn circle; using the 150× objective (Leica), the laser cut is ∼1 μm. 3, 4, A successful cut was controlled in the bright field and fluorescent mode. Scale bar, 20 μm. C, The accuracy of isolation was analyzed by RT-PCR using primers specific for the ubiquitously expressed cyclophilin (cyc), EYFP, the neuronal marker Thy-1, and CamKIIα. Otx-1 was chosen as a marker for cortical layer V projection neurons and GFAP as an astrocytic marker. All primers were located near the 3′ terminus. The cDNA template was generated from one round of linear amplified RNA. Total RNA was isolated from a pool of 100 EYFP-positive (Y) or 100 randomly selected EYFP-negative (N) cells from one TYNC cortex section not restricted to layer V. Lanes labeled with — are controls without cDNA. EYFP and Otx-1 mRNAs were detected in the YFP-positive pool of cells. The mRNA of markers for principal neurons Thy-1 and CamKIIα could be detected in the Y and N pools. GFAP expression was only detected in the EYFP-negative pool, suggesting that the contamination with cell material derived from astrocytes in the EYFP fraction is low. D, Two rounds amplified biotin-labeled RNA analyzed with the Bioanalyzer (Agilent). Shown are three independent samples of pools of 100 EYFP-positive cells isolated from layer V of the motor cortex (Y+MCx) and the somatosensory cortex (SSCx). The relative size distribution range is from 0.2 to >1 kB.