Abstract
Prefrontal neurons have been shown to represent task rules. Here we show the mechanisms by which the rule-selective activity in the prefrontal cortex influences subsequent cognitive performance based on that rule. Using functional magnetic resonance imaging, we found that the frontopolar cortex interacted with posterior areas differently depending on whether subjects were going to perform a phonological or semantic task. Moreover, we found that the sustained “set” activity in this region predicted the activity that could be recorded in the posterior areas during the performance, as well as the speed of that performance. We argue that the prefrontal set activity does not reflect simple maintenance of the task rules but the process of implementing the rule for subsequent cognitive performance and that this is done through rule-selective interactions with areas involved in execution of the tasks.
Keywords: prefrontal cortex, fMRI, cognition, connectivity, human, behavior
Introduction
Goal-directed behavior, future planning, and behavioral flexibility all depend on the ability of the brain to represent and implement the rules that guide behavior (Miller and Cohen, 2001). Previous studies have shown that the prefrontal cortex plays a key role in representing task rules (for review, see Bunge, 2004; Bunge et al., 2005). Rule-selective activity or inter-regional interactions has been identified in the prefrontal cortex just after the task instruction is given, before the actual performance of the task based on the rule (White and Wise, 1999; Asaad et al., 2000; Hoshi et al., 2000; Wallis et al., 2001; Bunge et al., 2003; Sakai and Passingham, 2003).
It remains open, however, how this preparatory or “task-set” activity guides subsequent task performance. The issue is significant given the report of prefrontal patients who can remember the rules but fail to implement the rules during the actual task performance (Burgess et al., 2000), which suggests that rule implementation can be dissociated from representing rules. This issue is also related to the functional significance of the rule-selective activity. Although neurons representing task rules are found in widely distributed prefrontal areas as well as in premotor and parietal areas (Wallis et al., 2001; Wallis and Miller, 2003; Stoet and Snyder, 2004), in a patient study, impairments in rule-based behavior were associated with lesions in the frontopolar cortex (FPC) (Burgess et al., 2000). Thus, the question remains as to whether there is or is not a critical subset of the task-set activity that is related to subsequent behavior. Behaviorally, the functional significance of the task-set activity can be shown as the presence of preparation effect; a very short delay between the instruction and task slows down the performance, and this has been thought of as attributable to insufficient time to establish the task operations before its actual performance (Rogers and Monsell, 1995; Monsell, 2003) (for other accounts, see Meiran et al., 2000; Wylie and Allport, 2000).
Here, by comparing between the tasks that show a preparation effect and a task without the effect, we show that a specific region of the prefrontal cortex, the FPC, is associated with this preparation effect. We further show that the task-set activity in the FPC is a significant predictor for the activity during the subsequent task performance in posterior prefrontal and premotor areas. We argue that rule-selective inter-regional interactions between the FPC and areas involved in task execution provide the mechanism by which task-set activity guides performance.
Materials and Methods
Subjects.
Fourteen normal, right-handed volunteers (age range of 21–36 years; seven males, seven females) gave written informed consent to participate in the study. All were native English speakers. The study was approved by the joint ethics committee of the Institute of Neurology and University College London Hospital (London, UK).
Behavioral task.
Subjects were required to make phonological, semantic, or visual judgments for a visually presented word. On presentation of a word, the subjects were asked to press a button with the right index finger as quickly as possible when the word had two syllables, had an abstract meaning, or was written in uppercase. They fixated on a white cross at the center of the screen throughout the experiment. Each task trial started with the presentation of the task instruction, “two-syllable, ” “abstract, ” or “uppercase, ” for 500 ms, 4° above the fixation cross. After an instruction delay of 0.3, 2, 4, 6, or 8 s, a target word was presented for 500 ms, 4° above the fixation cross, subtended <12° in horizontal axis. The words were nouns with written frequency over 30, chosen from the Medical Research Council Psycholinguistic Database (http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). Half of the words had two syllables, and the other half had one or three syllables. Half of the words had a concreteness rating below 300, and the other half had a rating above 550. This selection criterion was successful in equating the reaction time (RT) between the phonological and semantic tasks. Half of the words were written in uppercase and the other half in lowercase. Each possible combination of syllable, concreteness, and case dimensions was presented with equal frequency. The three types of task instruction were given in pseudorandom orders such that the same instruction was not repeated. Before the scanning, subjects performed a practice session for 15 min using 90 words, which included 30 trials for each task condition. During the scanning session, the total number of trials for each type of instruction was 40; thus, 120 trials were given using different words. The interval between the presentation of a task item and the task instruction for the next trial was varied from 4 to 8 s in a step of 2 s.
Functional magnetic resonance imaging.
Imaging was performed using a 1.5 tesla scanner (Sonata; Siemens, Erlagen, Germany). The functional images sensitive to blood oxygenation level-dependent (BOLD) contrasts were acquired by T2*-weighted echo planar imaging [repetition time (TR), 2.7 s; echo time (TE), 40 ms; 470 sequential whole-brain volume acquisition; in-plane resolution of 3 mm in 64 × 64 matrix; 30 slices; slice thickness of 4 mm; interslice gap 1 mm]. The onset of each task trial (task instruction) relative to the preceding image acquisition was jittered in steps of 540 ms within 1 TR (2.7 s). High-resolution structural T1-weighted magnetization-prepared rapid-acquisition gradient echo images (TR, 9.5 s; TE, 4 ms; inversion time, 600 ms; voxel size of 1 × 1 × 1.5 mm; 108 slices) were also acquired for all subjects.
Data analysis.
We used SPM2 software (http://www.fil.ion.ucl.ac.uk/spm) for image processing and analysis. The first five volumes of the images were discarded to allow for T1 equilibration. The remaining 465 image volumes were realigned to the first image, corrected for differences in slice acquisition timing, and normalized to the Montreal Neurological Institute (Montreal, Quebec, Canada) reference brain using a 12-parameter affine transformation along with a nonlinear transformation using cosine basis functions. The images were resampled into 2 mm cubic voxels and spatially smoothed with a Gaussian kernel (8 mm, full-width at half maximum). Statistical parametric maps of t statistics were calculated for condition-specific effects within a general linear model. For each condition, sustained activity during the instruction delay was modeled as an epoch with its onset time-locked to the presentation of the instruction and with duration matched to the length of the delay (Pho_set, Sem_set, and Vis_set). The model also included separate covariates for transient activation in response to the presentation for word stimuli separately for each condition (Pho_task, Sem_task, and Vis_task) and for transient activation in response to the presentation of instruction and key press commonly for the three conditions. Head-motion parameters in six dimensions were also included in the model as covariates of no interest. All epochs and events were convolved with a canonical hemodynamic response function. The data were high-pass filtered with a frequency cutoff at 100 s. We performed a random-effects analysis. Images of parameter estimates for the contrast of interest were created for each subject (first-level analysis) and were then entered into a second-level analysis using a one-sample t test across the 14 subjects. Based on our a priori hypothesis about the prefrontal cortex, we used a threshold of p < 0.05 corrected within the frontal convexity, which corresponds to t > 4.25.
Identification of activation foci.
First, we identified areas that were differentially active during the instruction delay depending on the conditions. We made comparisons between Pho_set and Vis_set and between Sem_set and Vis_set using F map of all set activity as an inclusive mask (p < 0.01, uncorrected). We also compared Pho_set and Sem_set. We next identified areas that showed phasic activity after presentation of a word. We made comparisons between Pho_task and Vis_task, between Sem_task and Vis_task, and between Pho_task and Sem_task.
Time course of activation.
The time series of the BOLD signals at the peak of activation was realigned at the onset of the word presentation and was resampled in 2 s time bins. The signals within each bin were then averaged across trials for the 14 subjects. This was done separately for trials with different length of the delay and separately for each task condition. For the activation in the FPC, we collected signals from the coordinate (−32, 56, 6), which was identified by the contrast: [(Pho_set) + (Sem_set)] − (Vis_set).
Correlation of activation between regions during the instruction delay.
We next examined the inter-regional interactions during the instruction delay by collecting the BOLD signals from the peak activation in the FPC, premotor cortex (PM), and anterior part of the ventrolateral prefrontal cortex (aVLPF). We did not shift the time window for analysis by the hemodynamic delay. Rather, we collected signals from the image volumes actually acquired during the instruction delay. This is because the aim of the analysis is to demonstrate the predictability of the kind of task to be performed based solely on the preparatory activity during the delay. Had we shifted the analysis window for the delay, the results on the delay activity could have been contaminated with activity related to the following task execution. Our results are free from the effect of task activity.
With the delay length of 2, 4, 6, and 8 s, TR of 2.7 s, and trial number of 8 for each delay length, the number of data points for the analysis was 60 for each condition, each subject. The amplitude of the BOLD signal differed because of different rise time across trials with different length of the instruction delay. Thus, there is a possibility that the difference in correlations of the BOLD signals across task conditions may be attributable to the delay lengths and time points within the delay. We removed the effect of delay lengths by adjusting the mean of the signals in a given delay length to zero separately for each of the 2 s time bins within the delay, and this was done for each subject and for each condition.
We then plotted the adjusted signals in the PM and aVLPF against the ones in the FPC and estimated the linear correlation of the signals, implicitly assigning the signals in the FPC as independent variables. For each condition and for each subject, we calculated the Pearson’s product-moment correlation coefficients r, which reflects the strength of inter-regional signal coupling. After normalizing the r using Fisher’s transformation, we performed an ANOVA with the three conditions as a within-subject factor.
Correlation of activation and reaction time.
We estimated linear correlation between the set activity in the FPC just before the presentation of a word (−2.7 to 0 s; note that we used TR of 2.7 s) and the RT of subsequent task performance. We removed the effect of the delay length by adjusting the mean of the BOLD signals in a given delay length to zero, and this was done separately for each subject and for each condition. We applied the same procedure to the RT data. Given the small amount of signal data available for trials with 0.3 s delay, we excluded them from the analysis. Thus, the analysis was based on the data for 32 trials for each condition, for each subject.
Multiple regression analysis.
Our hypothesis is that the task rules are implemented through interactions between areas that show task-set activity and areas involved in task execution. For this purpose, we collected the signal data during the set phase from −2.7 to 0 s before the onset of task items and also during task performance from 6 to 8.7 s after the onset of task items. The trials with instruction delay of 0.3 s were excluded from the analysis, and hence the number of data points was 32 (trials) × 14 (subjects). We performed multiple regression analysis to test whether the task activity can be predicted from the set activity. The task activity in the PM or aVLPF was assigned as a dependent variable, and set activity in the PM, aVLPF, FPC, and dorsolateral prefrontal cortex (DLPF) as well as the task activity in the fusiform gyrus (FG) were assigned as predictor variables. Before estimating the model, we factored out the effect of RT from the task activity in all areas. We also removed the effect of the delay length from the set activity as in the analysis of correlation of activation and RT. We used a forward-selection procedure to find out the predictor variables that were significantly correlated with the dependent variable. The variables were entered into the equation only when the significance level, F, was <0.05. The estimation procedures were conducted separately for phonological, semantic, and visual conditions.
Results
Behavioral data
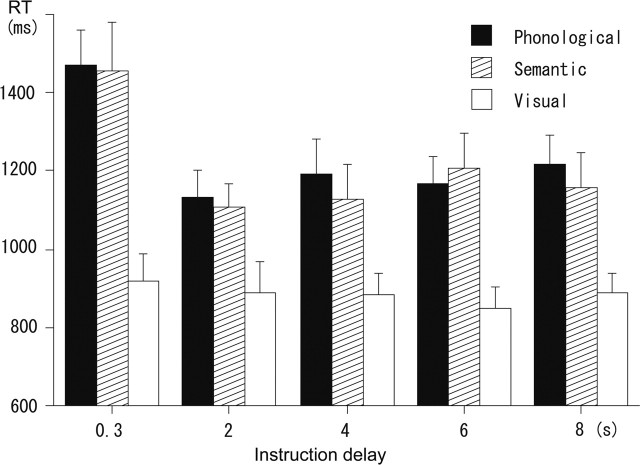
All subjects performed the tasks with high accuracy. The error rate was no more than 5% in all task conditions for all of the subjects (mean error rate, 2.1, 2.3, and 0.4% for phonological, semantic, and visual conditions, respectively). The RT of the subjects’ performance was significantly longer for the phonological and semantic conditions than for the visual condition [main effect of condition, F(2, 26) = 20.9, p < 0.01; post hoc analysis using Tukey’s honestly significant difference (HSD) test, p < 0.01] (Fig. 1). The RT did not differ significantly between the phonological and semantic conditions (p > 0.1). Notably, there was a significant interaction between the task condition and the length of the instruction delay (F(8, 104) = 3.53, p < 0.01). In the phonological and semantic conditions, the RT was significantly longer on trials with a delay length of 0.3 s than on trials with a longer delay (HSD test, p < 0.01), whereas in the visual condition, the RT did not differ significantly across the different delay lengths (p > 0.1). This interaction suggests that the difference between the phonological/semantic tasks and the visual task lies not just in the actual task performance but also in the preparatory processes. During the instruction delay, subjects are thought to be engaged in configuring a task set, and the delay in the performance on trials with short delay suggests the presence of an additional time-requiring process to prepare the phonological and semantic tasks compared with the visual task.
Figure 1.
RTs for the phonological, semantic, and visual tasks. The data are shown separately for trials with instruction delays of 0.3, 2, 4, 6, and 8 s.
Sustained set activity
In accordance with this preparation effect, we found additional neural activation in the phonological and semantic tasks compared with visual task. During the instruction delay, there was sustained activation in the lateral portion of the FPC (Brodmann’s area 10) that was significantly larger in the phonological and semantic conditions than in the visual condition (p < 0.05, small volume correction within the frontal convexity) (Fig. 2A; Table 1). The period of the sustained activity expanded and contracted according to the length of the instruction delay. Thus, the activity in the FPC may reflect the process of setting up and maintaining phonological and semantic task operations. There was also sustained activation in the middle frontal gyrus (DLPF, area 46) (p < 0.05) (Fig. 2A), but the activity did not differ significantly between the three conditions (p > 0.1).
Figure 2.
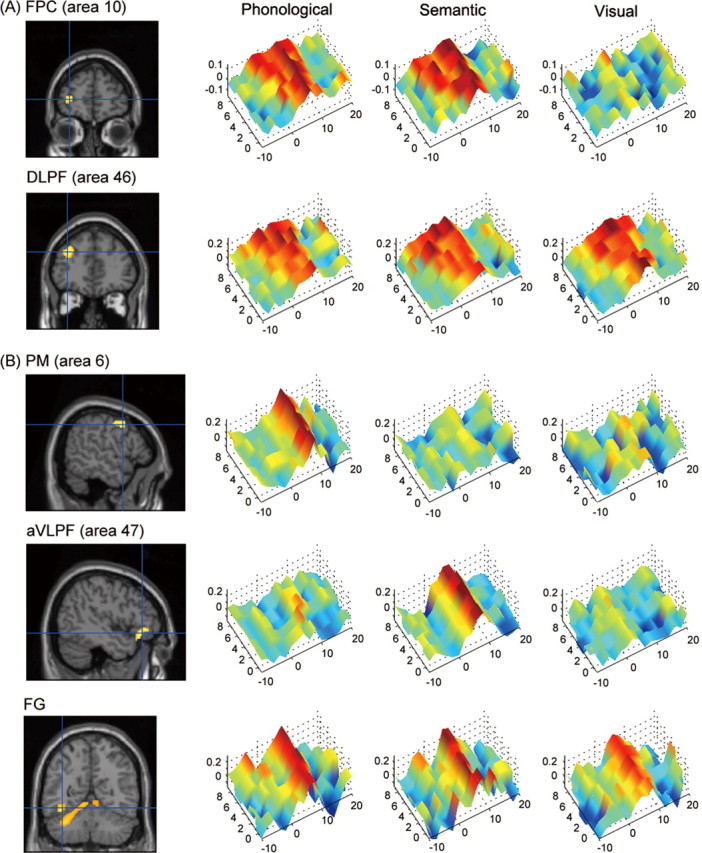
Areas that showed sustained activation during the pre-task period (A) and those that showed phasic activation during the task performance (B). A, The left FPC (area 10) and left DLPF (area 46). B, The left PM (area 6), left aVLPF (area 47), and the left FG. The peaks of the activation foci are shown as the blue crosshairs on the section images (left), and the time courses of the activity are shown as surface plots separately for the three conditions (right). The x-axis represents the time after the presentation of a target word (negative value indicates the time before the presentation) (in seconds), the y-axis represents the length of the instruction delay (in seconds), and z-axis represents the percentage increase of the BOLD signal with respect to the mean signal during the scan. The 0 on the y-axis corresponds to the instruction delay of 0.3 s.
Table 1.
Coordinates of the peak activation
| Contrast | Area | Coordinates | t value |
|---|---|---|---|
| Sustained activity during set | |||
| (Pho_set) > (Vis_set) | FPC (area 10) | (−30, 56, 4) | 4.48 |
| (Sem_set) > (Vis_set) | FPC (area 10) | (−32, 56, 8) | 4.70 |
| (Sem_set) > (Pho_set) | None | ||
| (Pho_set) > (Sem_set) | None | ||
| All_set | DLPF (area 46) | (−34, 44, 32) | 6.37 |
| ACC | (4, 28, 24) | 5.47 | |
| Lateral occipital | (−30, −94, −8) | 6.16 | |
| (34, −92, 0) | 6.72 | ||
| Phasic activity during task | |||
| (Pho_task) > (Vis_task) | pVLPF (area 44) | (−52, 12, 20) | 6.39 |
| (Sem_task) > (Vis_task) | pVLPF (area 44) | (−52, 20, 24) | 6.20 |
| aVLPF (area 47) | (−50, 34, −14) | 5.15 | |
| (Pho_task) > (Sem_task) | PM (area 6) | (−54, 6, 42) | 5.04 |
| (Sem_task) > (Pho_task) | aVLPF (area 47) | (−50, 34, −10) | 6.16 |
| MTG | (−54, −34, 2) | 4.29 | |
| All_task | FG | (−38, −56, −14) | 9.34 |
| Ventral occipital | (14, −86, −10) | 17.18 | |
| (−14, −84, −18) | 15.32 | ||
| (−8, −72, −8) | 11.55 | ||
| Cerebellum | (−34, −70, −22) | 12.96 | |
| pVLPF (area 44) | (−56, 14, 16) | 13.58 | |
| Inferior temporal | (50, −64, −14) | 10.18 |
We used a threshold of p < 0.05 corrected within the frontal convexity, which corresponds to t> 4.25. For All_task, areas with p < 0.05 corrected for the whole brain, which corresponds to t> 8.80, are shown. ACC, Anterior cingulate cortex; pVLPF, posterior VLPF; MTG, middle temporal gyrus.
Phasic task activity
During task performance, in contrast, the ventral part of the PM (area 6) was significantly more active in the phonological than semantic condition (p < 0.05), whereas the aVLPF (area 47) was significantly more active in the semantic than phonological condition (p < 0.05) (Fig. 2B). Activity in the posterior ventrolateral prefrontal cortex (area 44) was significantly larger in both the phonological and semantic conditions than in the visual condition (p < 0.05). The findings are consistent with previous studies showing separate but overlapping neural mechanisms for phonological and semantic processing (Poldrack et al., 1999; Wagner et al., 2000; Devlin et al., 2003). We also found that the visual areas, including the left mid-FG, were active in all task conditions (Fig. 2B), which may reflect an early stage of visual word processing (Cohen et al., 2002; Price and Devlin, 2003).
Rule-selective neural interactions
As above, there was no area in which the amount of pre-task activity differed between the phonological and semantic conditions. How then is the task rule represented during the instruction delay? We found that the interactions between the FPC and PM and those between the FPC and aVLPF differed according to which task the subjects were to perform. We calculated the correlation of the activity during the instruction delay between these regions. We found that the normalized correlation coefficient between the FPC and PM was significantly larger in the phonological than in the semantic and visual conditions (main effect of condition, F(2, 26) = 6.6, p < 0.05; post hoc analysis using HSD test, p < 0.05) (Fig. 3A, left). In contrast, the correlation of the activity between the FPC and aVLPF was significantly larger in the semantic than in the phonological and visual conditions (F(2, 26) = 4.0, p < 0.05; HSD test, p < 0.05) (Fig. 3A, right). Note that we can make comparisons only between different conditions in a given pair of regions, and not between different pairs of regions in a given task condition, because the effect of spatial smoothing and hence the correlation of activity could depend on the distance between the pair of regions.
Figure 3.
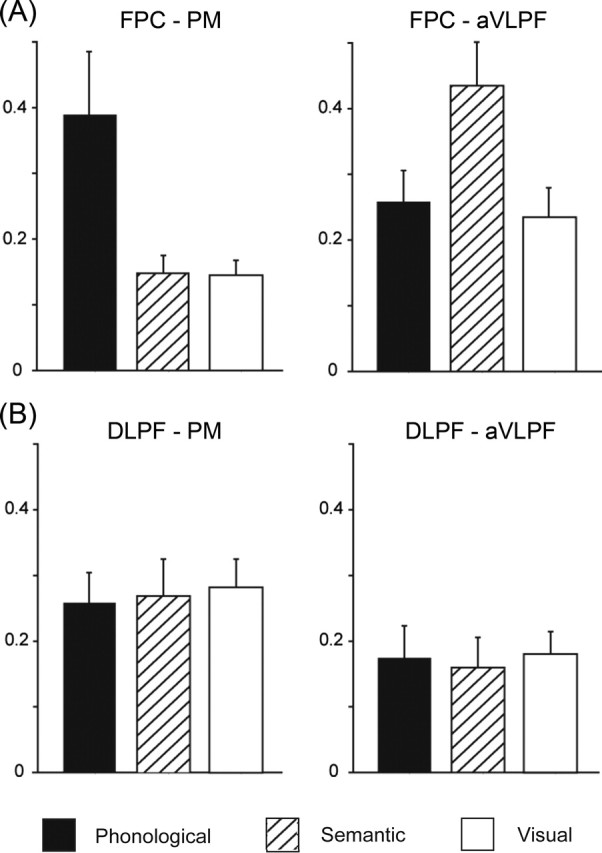
Inter-regional interaction during the instruction delay. A, Correlation of signals between the FPC and PM (left) and between the FPC and aVLPF (right). B, Correlation of signals between the DLPF and PM (left) and between the DLPF and aVLPF (right). The across-subject means of the normalized correlation coefficients r values are plotted separately for the three task conditions.
The task-selective increase in the inter-regional interactions during the set phase was specific to the FPC. Although there also was sustained activity during the instruction delay in the DLPF, the correlation with the PM or aVLPF did not differ between the phonological and semantic conditions (F(2, 26) = 0.16 and 0.11 for PM and aVLPF, respectively, p > 0.1) (Fig. 3B). The finding suggests that the FPC is specifically involved in distinguishing between the task rules before task performance.
Prediction of task performance
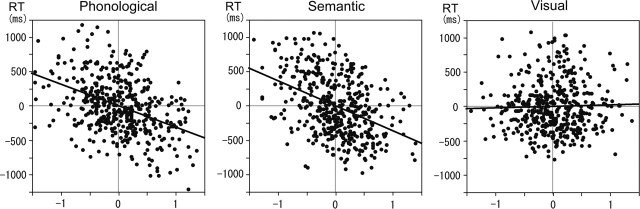
We found that the pre-task-set activity in the FPC predicted the RT of the subsequent task performance. Having removed the effect of the delay length from the set activity in the FPC, we still found that the activity was inversely correlated with the RT (Fig. 4). This relationship was significant in the phonological and semantic conditions (phonological condition, F(1, 446) = 90.6, r = 0.41, p < 0.001; semantic condition, F(1, 446) = 101.7, r = 0.43, p < 0.001) but not in visual condition (F(1, 446) = 0.37, r = 0.03, p > 0.1), suggesting that the role of the FPC is specific to the preparation of phonological and semantic judgment.
Figure 4.
Correlation between the set activity in the FPC and the RT for the three task conditions. The activity is shown as percentage signal change from the mean. The thick oblique line in each panel indicates the estimated linear regression line. Because of the mean adjustment to account for the effect of different delay lengths, the mean values for both the RT and FPC activity in the figures are zero.
We confirmed the finding by assigning the subject factor as a random effect. The standardized coefficient, β, was calculated for the regression analysis for each subject, and a one-sample t test was performed on the β values across the 14 subjects. We found that the β values were significantly less than zero in the phonological and semantic conditions, indicating negative correlations (phonological condition, t(1, 13) = −7.94, p < 0.001; semantic condition, t(1, 13) = −7.38, p < 0.001). The β values in the visual condition were not significantly different from zero (t(1, 13) = 0.45, p > 0.1).
Prediction of task-related activity
We further clarified the mechanisms by which the rule-selective set activity/interactions influence subsequent task performance. Our hypothesis is that a task rule is implemented through interactions with regions that are specifically involved in the task performance based on that rule. Consistent with the hypothesis, we found that the set activity in the FPC was correlated with the task activity in the PM and aVLPF. The correlation was negative and task selective: the set activity in the FPC was inversely correlated with the activity in the PM during performance of the phonological task (p < 0.0001), but the correlation between the pair was not significant in other tasks (p > 0.1). Conversely, the set activity in the FPC was inversely correlated with the activity in the aVLPF during performance of the semantic task (p < 0.0001), but no correlation was observed in other tasks (p > 0.1).
To demonstrate that the FPC plays a specific role in implementing task rules, we further conducted a multiple regression analysis by using, as predictor variables, the set activity in the DLPF, PM, and aVLPF in addition to the set activity in the FPC. We also added the task activity in the left FG as a predictor variable because this area is thought to be involved in the initial stage of visual word processing and thus is situated upstream to the PM and aVLPF. The analysis was performed separately for the task activity in the PM and aVLPF as the dependent variable. Before estimating the model, we factored out the effect of RT from the task activity and the effect of the instruction delay length from the set activity. Using a forward-selection procedure, we found that the task activity in the FG had the largest positive contribution to the task activity in the PM and aVLPF. However, the effect was not specific to the task: the activity in this area was positively correlated with activity in the PM and aVLPF in all the three conditions (when the dependent variable is the task activity in the PM, F(1, 446) = 103.8, 65.8, and 74.0 for phonological, semantic, and visual conditions, respectively, p < 0.0001; when the dependent variable is the task activity in the aVLPF, F(1, 446) = 72.6, 98.8, and 103.2 for phonological, semantic, and visual conditions, respectively, p < 0.0001) (Fig. 5A, B, top rows; Table 2). The set activity in the FPC showed the next largest correlation. After removing the effect of the activity in the FG, we still found task-selective and negative effect of the FPC. Only in the phonological condition was there a significant negative correlation between the set activity in the FPC and task activity in the PM (F(2, 445) = 98.5, p < 0.0001 for phonological) (Fig. 5A, bottom row). Only in the semantic condition was there a significant negative correlation between the set activity in the FPC and task activity in the aVLPF (F(2, 445) = 93.9, p < 0.0001 for semantic) (Fig. 5B, bottom row). The set activity in the DLPF, PM, and aVLPF did not predict the task activity in the PM and aVLPF (Table 2).
Figure 5.
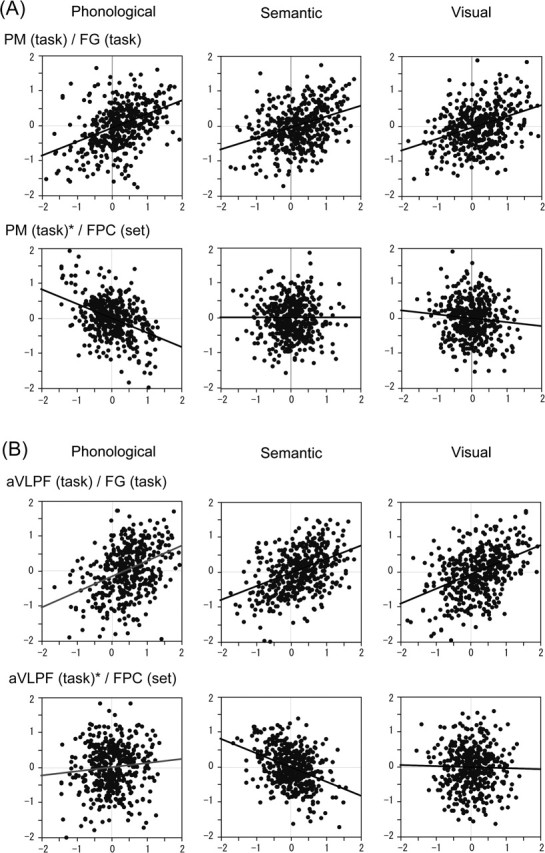
Multiple regression analysis with task activity in the PM (A) and aVLPF (B) as dependent variables. The three panels in the top row for A and B show the results with the task activity in the FG as the predictor variable. For the three panels in the bottom row of each figure, the dependent variables are the task activity in the PM or aVLPF after the effect of the FG has been factored out, and the predictor variable is the set activity in the FPC. The activity is shown as percentage signal change from the mean. The thick oblique line in each panel indicates the estimated linear regression line.
Table 2.
Standardized coefficients, β, of the multiple regression analysis
| Predictor variables | FG (task) | FPC (set) | DLPF (set) | PM (set) | aVLPF (set) |
|---|---|---|---|---|---|
| Dependent variable: PM (task) | |||||
| Phonological | 0.415 | −0.344 | −0.037 | −0.064 | −0.004 |
| (10.5) | (−8.70) | (−0.91) | (−1.57) | (−0.98) | |
| Semantic | 0.359 | −0.002 | 0.015 | 0.001 | 0.047 |
| (8.11) | (−0.05) | (0.34) | (0.02) | (1.05) | |
| Visual | 0.377 | −0.080 | −0.002 | −0.059 | 0.004 |
| (8.60) | (−1.81) | (−0.05) | (−1.35) | (0.10) | |
| Dependent variable: aVLPF (task) | |||||
| Phonological | 0.374 | 0.056 | 0.003 | 0.069 | −0.042 |
| (8.52) | (1.28) | (0.06) | (1.57) | (−0.95) | |
| Semantic | 0.395 | −0.341 | −0.010 | −0.013 | −0.011 |
| (9.89) | (−8.54) | (−0.25) | (−0.32) | (−0.25) | |
| Visual | 0.434 | −0.025 | −0.039 | 0.072 | 0.044 |
| (10.2) | (−0.59) | (−0.92) | (1.70) | (1.03) | |
Values in the parentheses are the tvalues. The dependent variable is the task activity in the PM or aVLPF, and the predictor variables are the task activity in the FG and the set activity in the FPC, DLPF, PM, and aVLPF. Values in bold are significant at p < 0.001. Other values did not reach significance (p > 0.1).
We next confirmed the finding by assigning the subject factor as a random effect. For each subject, we performed a regression analysis and calculated the standardized coefficient, β. Here the dependent variables are the task activity in the PM and aVLPF after the effect of the FG has been factored out, and the predictor variable is the set activity in the FPC. As for the regression between the task activity in the PM and the set activity in the FPC, we found that the β values were significantly less than zero only in the phonological condition (phonological condition, t(1, 13) = −9.90, p < 0.001; semantic condition, t(1, 13) = −0.23, p > 0.05; visual condition, t(1, 13) = −1.27, p > 0.05). As for the regression between the task activity in the aVLPF and the set activity in the FPC, we found that the β values were significantly less than zero only in the semantic condition (phonological condition, t(1, 13) = 1.88, p > 0.05; semantic condition, t(1, 13) = −8.37, p < 0.001; visual condition, t(1, 13) = −0.09, p > 0.05).
Thus, the on-line task processing, which is expressed by the activity in the task-specific posterior frontal areas, can be predicted by the set activity in the FPC and task activity in the FG. Whereas the activity in the FG, which reflects visual processing of the words, influenced the task processing in a nonspecific manner, the activity in the FPC, which reflects endogenous signals related to task rule, influenced the task processing in a task-specific manner. We argue that this is the way the task rule is implemented.
Discussion
Set activity in the FPC
We have shown rule-selective patterns of inter-regional interactions before the actual performance of the task. In particular, our results indicate the importance of the FPC in representing rules of the to-be-performed task. However, the question arises as to why we found rule-specific interactions for the phonological and semantic judgments but not for the visual judgment of a word. The phonological judgment requires transformation of the visual code (visually presented word) into a phonological code. The semantic judgment requires transformation of the visual code into a conceptual code. In contrast, the visual case judgment does not require any code transformation: subjects simply discriminate between the visual features. Thus, the FPC may be involved in setting up task operations that require active manipulation of the items such as transformation between codes. The finding counters the possibility that the set activity in this region reflects nonspecific arousal or verbal rehearsal of the task instructions.
The rule-specific patterns of interactions between the FPC and the posterior frontal areas are consistent with our previous study, in which the FPC interacted with posterior superior frontal sulcus (area 8) or posterior inferior frontal gyrus (area 44), depending on whether the subjects were to perform a spatial or verbal working memory task (Sakai and Passingham, 2003). Of note is that both tasks involved transformation of a sensory code into a motor code: registering a spatial sequence involves covert shifts of attention, and registering a letter sequence involves covert vocalization (Passingham and Sakai, 2004). In that study, the inter-regional correlation increased when subjects prepared to reorder the task items. However, it could be argued that this simply reflected differences in task difficulty. The present study is not open to that objection because we found that the semantic and phonological tasks were of equal difficulty, as judged by the RTs. Furthermore, the FPC interacted with different areas although the operations were to be performed on the same task items.
In the present study, we found the peak on the left side, anterior to the frontomarginal sulcus. In contrast, in our previous study using working memory tasks, the anterior prefrontal peak was observed on the right side and was posterior to the present one (Sakai and Passingham, 2003). It could be that this is because the rules of the tasks used in the present study were purely verbal. However, Bunge et al. (2003) observed a peak on the left side as in the present study when subjects prepared to perform a nonmatch task for nonverbal items, which the authors interpret as reflecting elaboration of a default “match” rule. Also, the FPC on both sides has been shown to be involved in holding multiple task rules (Koechlin et al., 1999; Burgess et al., 2003; Badre and Wagner, 2004). Of note is that the lesions in the left FPC have been shown to cause frequent rule breaking during performance of multiple tasks (Burgess et al., 2000). Additional studies are required to determine the functional difference between the left and right and between the anterior and posterior portion of the lateral FPC. Also, it remains open how the present finding can be explained in terms of the account that the FPC plays a role in integration of the outcomes of multiple cognitive operations (Christoff and Gabrieli, 2000; Ramnani and Owen, 2004). It could be that the integration is not a prerequisite for the recruitment of the FPC or some integration process takes place during preparation for a task that requires manipulation of task items.
Set activity in DLPF
The DLPF showed sustained pre-task activity not only in the phonological and semantic tasks but also in the visual task. This activity could reflect maintenance of task instruction regardless of the nature of the tasks, but it is unlikely that the information is maintained in verbal/phonological forms because the left ventrolateral prefrontal cortex did not show sustained activity. The nonspecific involvement of the DLPF in rule maintenance is consistent with Bunge et al. (2003). One possibility is that the sustained pre-task activity in the DLPF may reflect active maintenance process in the face of across-trial interference caused by frequent switching between task instructions. Consistent with the idea, Sakai et al. (2002) have shown that the DLPF plays a critical role in active maintenance to counter the upcoming distractors. Although interference resolution has been associated with the ventrolateral prefrontal cortex (D’Esposito et al., 1999) (but see Sakai and Passingham, 2004), this ventral region shows phasic activity when subjects make response against the pre-potent bias. In contrast, the DLPF shows sustained activity. In the present study, this could reflect the maintenance of the currently active task rule against proactive interference from rules used in previous trials (Meiran et al., 2000; Wylie and Allport, 2000). The activity in the DLPF did not predict the speed of task performance. It could be correlated with the accuracy of task performance (Sakai et al., 2002).
Set activity in FPC predicts subsequent task performance
The correlation between the pre-task-set activity in the FPC and the RT suggests a causal link between the rule representation and the performance based on the rule. Although neuronal activity representing task rules can be found all over the prefrontal cortex (Wallis et al., 2001), our results suggest that, at least in the human brain, the FPC may have a specialized role in preparation of tasks that requires item manipulation.
The mechanism of task preparation has also been studied using a task-switching paradigm. These studies have shown that a region at the posterior end of the inferior frontal sulcus is specifically active when a cue indicates a switch of the task rules (for review, see Brass et al., 2005). In contrast to the rather phasic involvement of this area in reconfiguring task sets, the frontopolar cortex may exert a tonic influence over the task performance by maintaining task sets. In fact, it has been shown that the FPC is involved in sustained cognitive control during task switching (Braver et al., 2003) or in resolution of prolonged proactive interference from a previous cognitive set in the Wisconsin Card Sorting Test (Konishi et al., 2005).
Set activity in FPC predicts activity in posterior areas during task performance
We extended the previous studies of our own and others significantly by showing the mechanisms with which the sustained set activity that represents the rule influences the subsequent task performance. The set activity in the FPC is a significant determinant of subsequent activity during task performance in posterior areas. Importantly, this effect was specific to the rules of the task. This is in sharp contrast to the activity in areas involved in visual processing of the items, FG, the effect of which was nonspecific to the task rule. Thus, the performance of cognitive task is determined by the tonic endogenous drive reflecting the current task rule and phasic external inputs about the task items (Otten et al., 2002; Braver et al., 2003; Burgund et al., 2005).
Braver et al. (2003) found a negative correlation of activity in each of the FPC, DLPF, and ventrolateral prefrontal cortex between the pre-task period and task performance period that is an autocorrelation within a region. Kerns et al. (2004), using the Stroop task, have also shown correlation between the activity in the anterior cingulate cortex in a previous trial and the activity in the prefrontal cortex in the current trial. Our results are a significant advance on these in that they demonstrate the rule-selective change in the patterns of correlation. The task rule at hand determines whether the task activity in the PM or aVLPF is influenced by the set activity in the FPC. This could be mediated by the positive influence of the FPC over these areas during the pre-task period. During the pre-task period, the FPC may have primed the areas involved in task execution through positive and task-specific interactions, thereby reducing the on-line task-processing load before the task. High set activity in the FPC may lead to better configuration of the task operation in areas involved in task execution before task performance, and this could lead to less activation in those areas during the task performance because there is no need to set up the task operation after the presentation of task items.
In summary, our results suggest that the FPC is involved in the maintenance of task rules through rule-specific interactions with areas related to the task performance. Of note here is that what is maintained during the instruction delay is not the sensory information given in the past but rather the information generated for prospective use (Tanji and Hoshi, 2001; Passingham and Sakai, 2004). The predictive nature of the set activity in the FPC for the task performance/activity further suggests that these rule-selective interactions operate as the process of implementing the rule for subsequent cognitive performance. We argue that this is the way the prefrontal cortex prospectively configures and facilitates rule-based behavior.
Footnotes
This work was supported by a grant from the Wellcome Trust. K.S. is supported by the Career Development Award from the Human Frontier Science Program and by Grant-in-Aid 17022016 for Scientific Research on Priority Areas—System Study on Higher-Order Brain Functions from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Asaad WF, Rainer G, Miller EK (2000). Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol 84:451–459. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD (2004). Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron 41:473–487. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY (2005). The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9:314–316. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI (2003). Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39:713–726. [DOI] [PubMed] [Google Scholar]
- Bunge SA (2004). How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci 4:564–579. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD (2003). Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol 90:3419–3428. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai K (2005). Neural circuitry underlying rule use in humans and non-human primates. J Neurosci 25:10347–10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T (2000). The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 38:848–863. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD (2003). The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia 41:906–918. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Lugar HM, Schlaggar BL, Petersen SE (2005). Task demands modulate sustained and transient neural activity during visual-matching tasks. NeuroImage 25:511–519. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE (2000). The fronto-polar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychophysiology 28:168–186. [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S (2002). Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125:1054–1069. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE (1999). The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci USA 96:7514–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF (2003). Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci 15:71–84. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J (2000). Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol 83:2355–2373. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. Science 303:1023–1026. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J (1999). The role of the anterior prefrontal cortex in human cognition. Nature 399:148–151. [DOI] [PubMed] [Google Scholar]
- Konishi S, Chikazoe J, Jimura K, Asari T, Miyashita Y (2005). Neural mechanism in anterior prefrontal cortex for inhibition of prolonged set interference. Proc Natl Acad Sci USA 102:12584–12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N, Chorev Z, Sapir A (2000). Component processes in task switching. Cognit Psychol 41:211–253. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001). An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Monsell S (2003). Task switching. Trend Cogn Sci 7:134–140. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD (2002). State-related and item-related neural correlates of successful memory encoding. Nat Neurosci 5:1339–1344. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K (2004). The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol 14:163–168. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10:15–35. [DOI] [PubMed] [Google Scholar]
- Price C, Devlin JT (2003). The myth of the visual word form area. NeuroImage 19:473–481. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM (2004). Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5:184–194. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S (1995). Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124:207–231. [Google Scholar]
- Sakai K, Passingham RE (2003). Prefrontal interactions reflect future task operations. Nat Neurosci 6:75–81. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE (2004). Prefrontal selection and medial temporal lobe reactivation in retrieval of short-term verbal information. Cereb Cortex 14:914–921. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE (2002). Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci 5:479–484. [DOI] [PubMed] [Google Scholar]
- Stoet G, Snyder LH (2004). Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron 42:1003–1012. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E (2001). Behavioral planning in the prefrontal cortex. Curr Opin Neurobiol 11:164–170. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL (2000). Task-specific repetition priming in left inferior prefrontal cortex. Cereb Cortex 10:1176–1184. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK (2003). From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol 90:1790–1806. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK (2001). Single neurons in prefrontal cortex encode abstract rules. Nature 411:953–956. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP (1999). Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 126:315–335. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A (2000). Task switching and the measurement of “switch costs.”. Psychol Res 63:212–233. [DOI] [PubMed] [Google Scholar]