Figure 1.
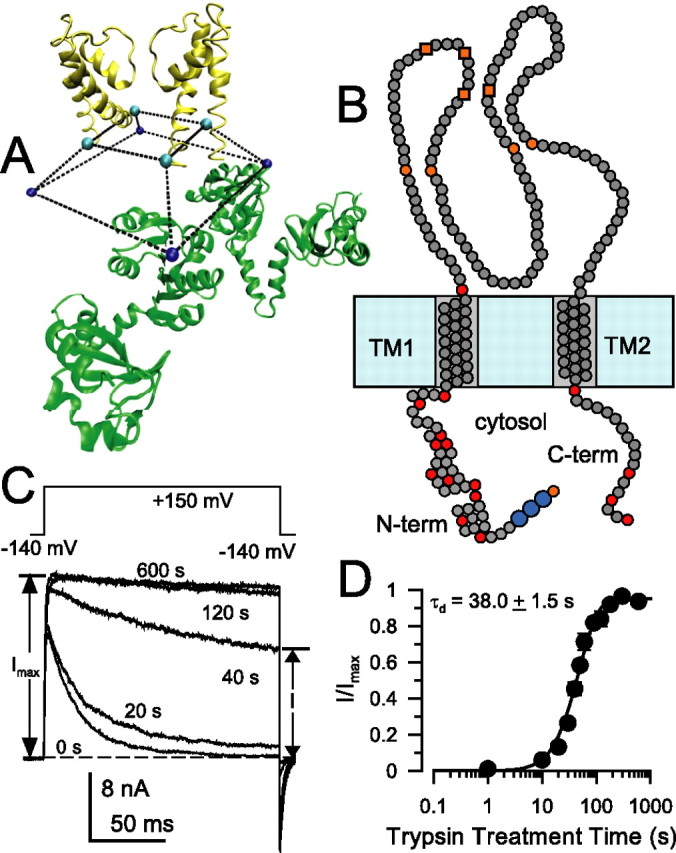
Characteristics of trypsin sensitivity of inactivation mediated by wild-type β2 N termini. A, The architecture of the cytosolic domain and pore domain of the MthK channel illustrates the idea that access of permeant ions and inactivation domains to the permeation pathway in many channels may occur via side portals. Here, two of the four MthK subunits are shown (inner RCK domains only), and the truncated square pyramid defines the volume delimited by the last residues in S6 (H90 in MthK) and first residues in the RCK domain. The specific structure of the linker connecting these residues is unknown. The distance between residues in different S6 helices is 26 Å. B, β2 subunits contain two transmembrane segments (TM1 and TM2), an extracellular loop with eight cysteine residues (4 orange circles correspond to cysteines shared with β1, β3, and β4 subunits; 4 orange boxes correspond to cysteines shared with β3 and β4), and a cytosolic N and C terminus. Basic residues on the cytosolic side are given as red circles, and basic residues delimiting transmembrane segments are also indicated. The larger blue circles indicate the FIW motif essential for β2 inactivation. C, Removal of inactivation by 0.1 mg/ml trypsin in wild-type α + β2 currents is essentially complete within ∼100 s. Currents were monitored by the indicated protocol with voltage steps to +150 mV with 10 μm Ca2+. Trypsin was applied for timed periods only between test steps. Times indicate cumulative periods of trypsin application. D, The fractional restoration of non-inactivating steady-state current from traces as in C is plotted as a function of trypsin (0.1 mg/ml) treatment time for a set of five patches. Recovery time course was fit with Equation 1, yielding a digestion time constant of τd = 38.0 ± 1.5 s, with n constrained to 2.0.
