Figure 7.
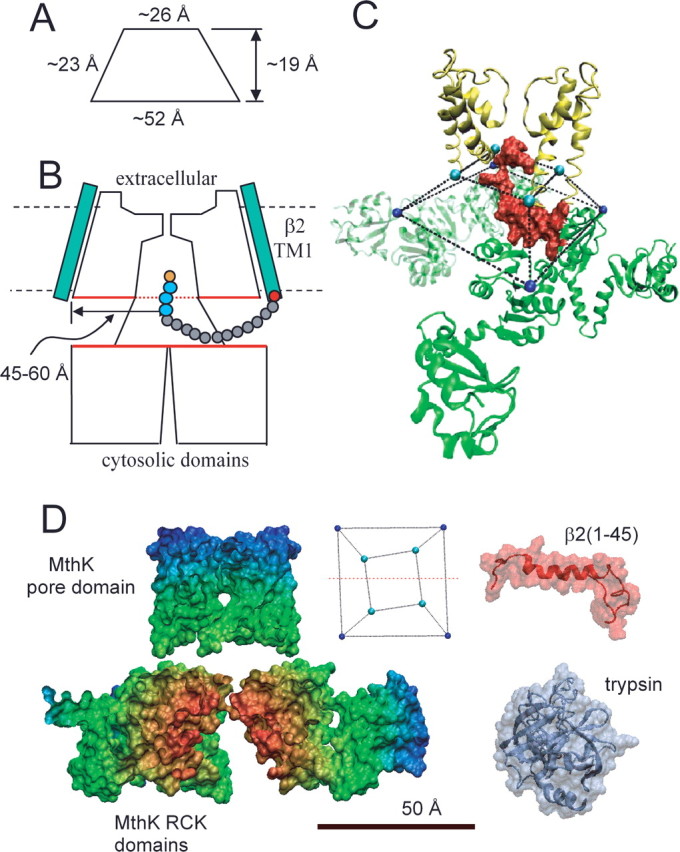
Considerations regarding the antechamber dimensions. A, The approximate dimensions of the truncated pyramid (side view) defined by residues at the end of S6 and beginning of RCK1 in MthK are given. B, A diagram of a possible relationship of a BK α subunit and the β2 subunit is shown. The β2 subunit is shown with a 16 amino acid N terminus ending with the MFIW inactivation motif (orange and blue residues). This 16 residue length defines the minimum necessary to permit any detectable inactivation (Xia et al., 2003), a length identical to that protected from digestion by trypsin. This suggests that the protected antechamber is larger than the volume circumscribed by the linkers between the S6 helices and the beginning of the cytosolic domains. The red boundaries of the pore domains and cytosolic domains denote the potential protected volume. The estimated distance from the axis of the permeation pathway to the edge of the protected disk is ∼45–60 Å. C, The initial 26 residues of the β2 N terminus are positioned within the volume circumscribed by the S6-to-RCK linkers, suggesting that a single inactivation domain is sufficiently to occupy the most of the antechamber. D, Comparison of space-filling structural models of MthK, the β2 N terminus (residues 1–45), and porcine pancreatic trypsin. For MthK, only residues on one side of a plane containing the axis of the permeation pathway (in inset, red line through truncated square pyramid defines the cut through the MthK structure) are shown. For β2, the underlying chain morphology is shown to emphasize the underlying flexibility that is probably essential to its action. The dimensions of trypsin exclude it from the compartment that separates the pore and cytosolic domains.
