Figure 3.
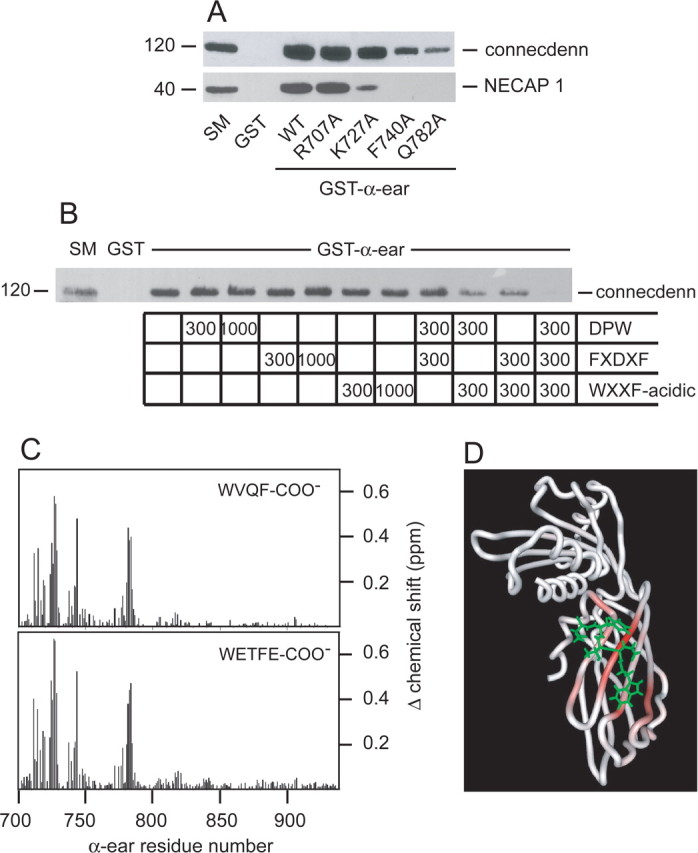
Identification of the connecdenn-binding site on AP-2. A, Soluble brain lysates were incubated with GST or GST fused to wild-type (WT) α-ear or a variety of α-ear point mutants as indicated, precoupled to glutathione-Sepharose beads. Proteins specifically bound to the beads were processed for Western blot with antibodies against the indicated proteins. B, Soluble brain lysates were incubated with GST or GST–α-ear, precoupled to glutathione-Sepharose beads. Incubations were without or with a DPW peptide (CSDPWGSDPWG) from epsin 1, a FXDXF peptide (CSFFEDNFVPE) from amphiphysin1, or a WXXF-acidic peptide (CQAPQPSNWVQF-COO−) from NECAP 1 at the molar ratios of peptide to fusion protein indicated. Proteins specifically bound to the beads were processed for Western blot with antibody against connecdenn. For all above experiments, an aliquot of starting material (SM) equal to 1/10 of that added to the beads was run in parallel. C, Magnitude of the amide chemical shift changes [{(Δ1H shift)2 + (Δ15N shift × 0.2)2}1/2 in parts per million (ppm)] of the α-ear during binding CQAPQPSNWVQF-COO− (WVQF-COO−, NECAP 1; top) and CVEQLRRQWETFE (WETFE-COO−, connecdenn; bottom). The residue numbers correspond to mouse α-adaptin. D, HADDOCK modeled structure of WETFE/α-ear complex. The backbone trace of the α-ear is colored according to the size of the amide chemical shift changes during binding of the connecdenn peptide.
