Figure 7.
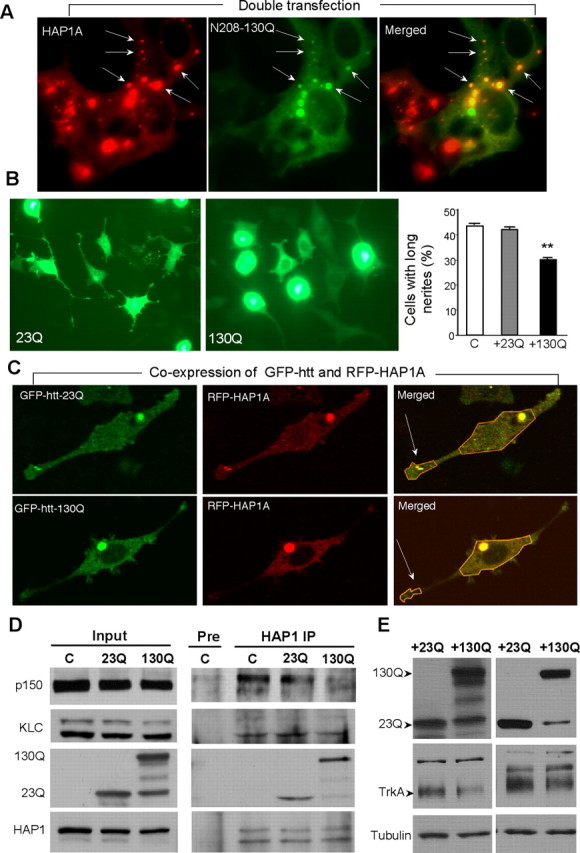
Mutant htt inhibits the association of HAP1 with trafficking proteins and the level of TrkA. A, HEK293 cells were cotransfected with HAP1A and N-terminal htt (1–208 aa) containing 130Q (130Q). Transfected mutant htt (130Q) is colocalized with HAP1A in some cytoplasmic puncta (arrows). Note that HAP1A itself also forms cytoplasmic puncta. B, PC12 cells were infected with adenoviral GFP-htt (1–208 aa) containing 23Q or 130Q and were treated with NGF (50 ng/ml for 48 h). Mutant htt (+130Q) inhibits neurite outgrowth compared with normal htt (+23Q). The percentage of PC12 cells containing neurites longer than two cell bodies was determined (right). C, Noninfected PC12 cells. Data (mean ± SE) were obtained from three experiments. ∗∗p < 0.01. C, Expression of GFP-htt-130Q reduced the distribution of RFP-HAP1A in neurite tips (arrows) of PC12 cells. Merged images show colocalized RFP-HAP1A and GFP-htt (yellow signal). Also see live-cell imaging in supplemental movies (available at www.jneurosci.org as supplemental material). D, Immunoprecipitation (IP) of HAP1A and its associated proteins in PC12 cells infected with adenoviral GFP-htt for 48 h. Mutant N-terminal htt (130Q) bound more HAP1 than did control htt (23Q) and resulted in decreased association of endogenous HAP1 with dynactin p150 and kinesin light chain in PC12 cells. Note that small or degraded protein products in the 130Q sample did not bind HAP1. The same HAP1 immunoprecipitates and input were probed with a panel of different antibodies as indicated. E, PC12 cells infected with adenoviral htt-23Q or 130Q were subjected to Western blotting. Mutant htt (130Q) reduced the level of TrkA in PC12 cells compared with wild-type htt (23Q). Results from two independent experiments are shown.
