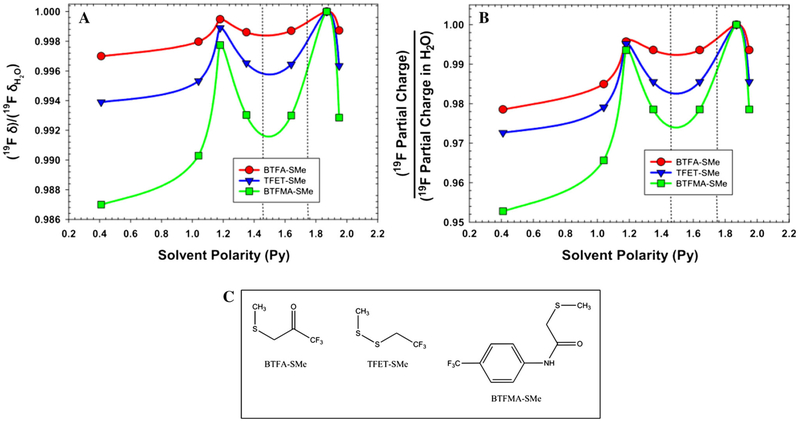
Fig. 3.
a DFT-based normalized chemical shifts for three CF3 tags conjugated to methanethiol (shown in c). Isotropic NMR shifts for each of the tags were calculated using the B3LYP hybrid functional and the 6-31G(d,p) basis set, under six different solvent conditions and one vapor phase [left to right: vapor phase (Py = 0.4), toluene (Py = 1.04), ethanol (Py = 1.18), methylene chloride (Py = 1.35), acetone (Py = 1.64), water (Py = 1.87), and dimethyl sulfoxide (Py = 1.95)]. b DFT-based normalized average partial charges for 19F atoms for each of the above three CF3 tags, as a function of solvent polarity. The partial charges for each of the 19F atoms in the CF3 group were averaged assuming isotropic conditions and fast methyl rotations and then normalized to the average 19F partial charge for the conjugate in H2O. Dotted lines indicate the polarity region assessed experimentally by NMR via MeOH/H2O solvent mixtures (Fig. 2). c Structure of the three methanethiol conjugates for which DFT calculations were performed