Figure 1. 6w-MSCs with RSV induction preserve the proliferative capacity even after reaching effective cell dose (ECD) for MSC therapy.
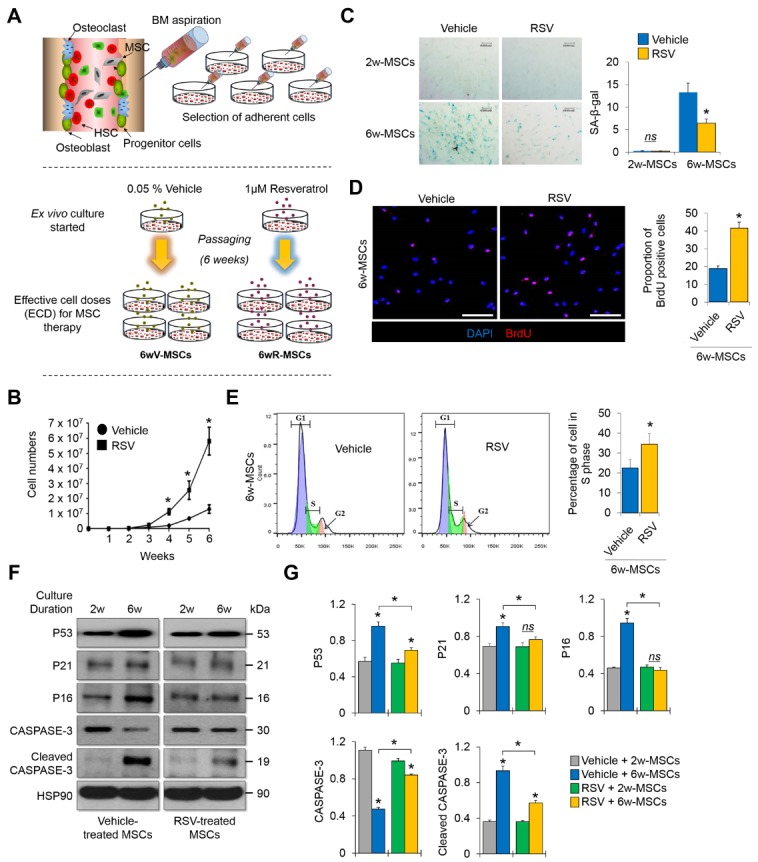
(A) Scheme for MSC isolation and long-term cultivation to obtain ECD-MSCs. First, 0.05% vehicle (EtOH) or 1 μM RSV was added to the medium for MSC culture and the vehicle or RSV-containing medium was exchanged every 2 days. The cells were cultured until vehicle-treated MSCs reached 1 × 107 cells, generally regarded as the ECD for bone regeneration. (B) The numbers of MSCs between 1-6 weeks of expansion in the absence or presence of RSV (n = 6 donors). The cells were counted every 7 days. (C) SA-β-gal assay was performed to compare cellular senescence between vehicle and RSV groups. 2w-MSCs refers to the MSCs cultured during 2 weeks from primary culture date, and 6w-MSCs refers to the MSCs cultured during 6 weeks from primary culture date in order to obtain 1 × 107 cells. SA-β-gal-positive cells were quantitated by ImageJ (n = 3, in triplicate per donor) (right). *p < 0.05 compared to vehicle-treated MSCs. 2w-MSCs, 3rd passage; 6w-MSCs, 13th passage. (D) Immunocytochemistry was performed to observe the bromodeoxyuridine-positive cell portion. Nuclei were stained with 4′,6-diamidino-2-phenylindole and images were captured by confocal microscopy. Scale bar = 100 µm. *, p < 0.05 compared to vehicle-treated 6w-MSCs (n = 3, in triplicate per donor). 6w-MSCs, 13th passage. (E) The proportion of 6w-MSCs treated with vehicle or RSV in each cell cycle phase was evaluated by flow cytometry with propidium iodide staining. *, p < 0.05 compared to vehicle-treated 6w-MSCs (n = 3, in triplicate per donor). 6w-MSCs, 13th passage. (F) Protein levels of P53, P21, P16, CASPASE-3, and cleaved CASPASE-3 were quantified by western blot analysis and normalized to that of HSP90. 2w-MSCs, 4th passage; 6w-MSCs, 13th passage. (G) Quantification of each protein level was determined by GraphPad Prism software (version 6.0). *, p < 0.05 compared to 2w- or vehicle-treated MSCs (n = 3, in triplicate per donor).
