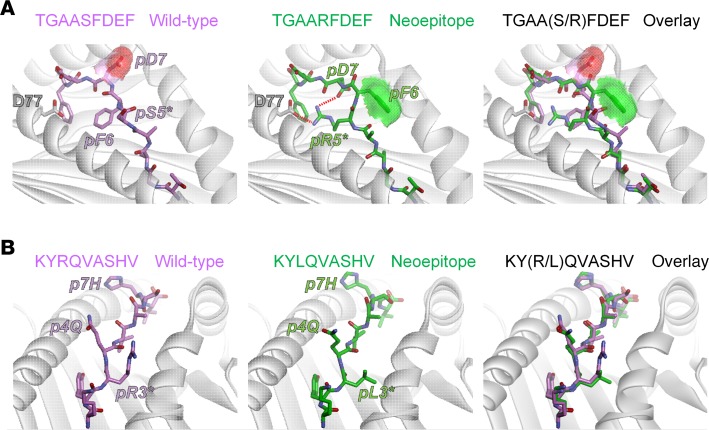
Figure 4. Models of peptide/MHC complexes indicate structural and physical correlates with immunogenicity.
(A) For the tumor rejecting TGAARFDEF neoepitope, the serine-to-arginine substitution at position 5 is predicted to alter peptide conformation and increase exposed hydrophobic surface area. In the model of the wild-type complex (left panel), Phe6 acts as a secondary anchor. Asp7 is solvent exposed, as indicated by the red surface. In the neoepitope complex (middle panel), the new arginine at p5 takes on the secondary anchor role, forming a salt-bridge with Asp77 of the H2-Dd α1 helix. The switch in secondary anchor from p6 to p5 results in Asp7 shifting down toward the base of the groove to form a second salt-bridge with Arg5, reducing its solvent exposure. Coincident with this shift, Phe6 moves up to become solvent exposed, as indicated by the green surface. An overlay of the neoepitope and its wild-type counterpart demonstrates the substantial differences between the two (right panel). (B) For the inactive KYLQVASHV neoepitope, the arginine-to-leucine substitution at position 3 does not alter peptide conformation. In the model of the wild-type complex (left panel), Arg3 is positioned between the peptide backbone and the H2-Kd α2 helix. Gln4 and His7 are exposed and face up toward incoming TCRs. In the neoepitope complex, the new leucine at p3 simply fills the same space between the peptide and the α2 helix and does not introduce any structural alterations to the peptide (middle panel). An overlay of the neoepitope and its wild-type counterpart demonstrates the similarities between the wild-type peptide and neoepitope.