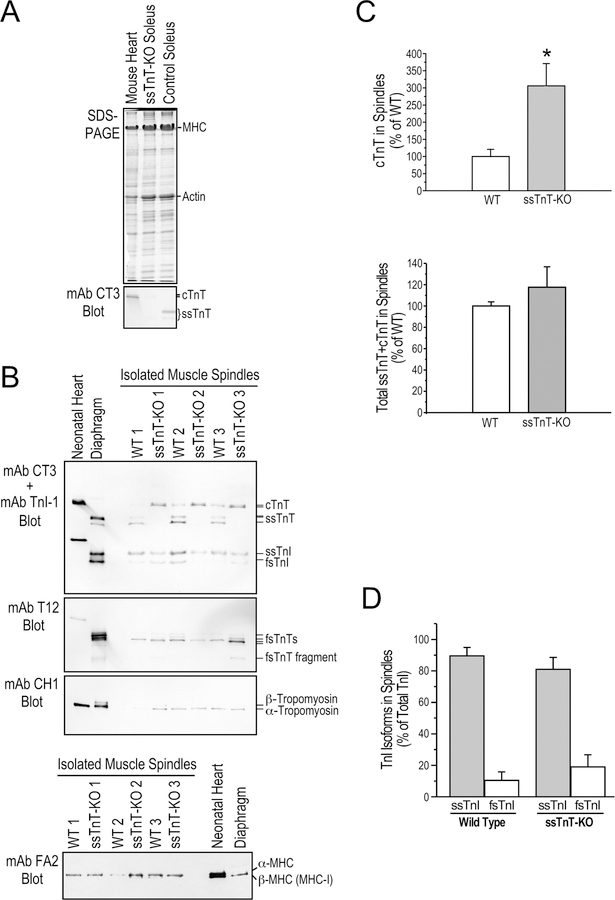
Figure 5. Altered myofilament protein contents in ssTnT-KO mouse spindle intrafusal fibers.
A. SDS-gel and mAb CT3 Western blot demonstrate the complete loss of ssTnT in Tnnt1-KO mouse soleus muscle with a detectable expression of cTnT reflecting the active regeneration (Wei et al., 2014). Normal mouse heart and soleus muscle were used as controls. B. Western blots of muscle spindle protein extracts from 3 WT and 3 ssTnT-KO mice using a mixture of mAbs CT3 and TnI-1 demonstrated significantly increased level of cTnT in the intrafusal fibers of ssTnT-KO mice while slow skeletal muscle TnI (ssTnI) remains the major form in the intrafusal fibers. mAb T12 blot detected a low molecular weight splice form as the major fsTnT in the intrafusal fibers of both WT and ssTnT-KO mice. mAb CH1 blot showed α-tropomyosin in the intrafusal fibers of both WT and ssTnT-KO mice. Neonatal mouse heart and adult diaphragm protein extracts were used as controls. The mAb FA2 Western blot in the lower panel showed no significant change in cardiac MHC isoform contents in ssTnT-KO spindle intrafusal fibers. C. Densitometry analysis quantified the significant increase of cTnT in ssTnT-KO muscle spindle intrafusal fibers, which completely compensated for the loss of ssTnT to produce a normal level of total cTnT+ssTnT comparable to WT controls. D. αDensitometry quantification confirmed that ssTnI remains the predominant isoform in ssTnT-KO muscle spindle intrafusal fibers. *P<0.05.