Abstract
The effect of the cell-free culture supernatants (CFCSs) from different Lacobacillus spp. on growth ability of Cronobacter sakazakii ATCC 29544 was investigated by time-killing studies. The antimicrobial effect was evaluated using crude and 2.5 × concentrated CFCSs. Most of the CFCSs showed a dose-dependent antimicrobial activity, with the greatest C. sakazakii growth inhibition exerted by the CFCS 2.5 × of Lactobacillus casei rhamnosus ATCC 7469. Indeed, C. sakazakii growth was completely inhibited after 4 h of incubation with the crude CFCSs of L. casei rhamnosus and Lactobacillus acidophilus and after only 2 h using the related 2.5 × CFCSs. The flow cytometric analysis revealed that CFCSs altered the permeability of C. sakazakii cell membrane, showing 55% of live cells after 30 min of treatment with 2.5 × CFCSs of L. casei rhamnosus and L. acidophilus, reaching 1% of live cells after 2 h of exposure. The CFCSs of L. casei rhamnosus and L. acidophilus have showed anti-Cronobacter activity, determining a progressively inhibition of C. sakazakii growth as result of alterations in its membrane permeability.
Keywords: Lactobacilli, Cell-free culture supernatants, Antimicrobial activity, C. sakazakii, Membrane integrity, Flow cytometry
Introduction
Cronobacter sakazakii is a food-borne pathogen associated with meningitis and necrotizing enterocolitis (NEC) in infants, septicemia and catheter-associated infections in elderly and immune-compromised people (Jason 2015). Although the low incidence of infection, mortality rate is significant, ranging from 30 to 80% and even, in case of survival, children are often affected by sepsis, meningitis, and brain abscess (Hunter and Bean 2013). C. sakazakii has been frequently isolated from powdered infant formula (PIF) (Li et al. 2016) that can be contaminated either by intrinsic contaminations occurring during the manufacturing process of PIF and extrinsic contaminations due to contaminated utensils (Jason 2015; Kalyantanda et al. 2015). The indication of the microbiological count of Enterobacteriaceae as a good indicator for risk of C. sakazakii has been withdrawn by the BIOHAZ Panel, that affirmed the “necessity of parallel testing for Enterobacteriaceae and C. sakazakii […]” (Commission Regulation-EC No 1441/2007).
Recently, there has been increased interest in the possible use of beneficial microorganisms, such as Lactic Acid Bacteria (LAB) as a biopreservation tool to inhibit the growth of foodborne pathogens in different food. The mechanism underlying the activity of LAB strains against bacterial pathogens appears to be multifactorial and include the production of hydrogen peroxide, lactic acid, bacteriocin-like molecules, stimulation of the immune system and modulation of intestinal microbiota (Salminen et al. 2010). The antimicrobial activity of LAB against a wide range of food-borne pathogens is well-established in different types of food products (Lin and Pan 2019; Lucera et al. 2012). Several studies have indicated that the growth of most common food pathogens, such as Listeria monocytogenes, Salmonella spp., Escherichia coli O157:H7, Staphylococcus aureus, can be controlled in ready-to-eat meat products by using LAB isolated either from raw meat or meat products (Aymerich et al. 2008; Teixeira et al. 2006). However, the antimicrobial activity of LAB against C. sakazakii is still poorly investigated. For this, the aim of this study was to examine the antimicrobial effect of the cell-free culture supernatants (CFCSs) obtained from different Lactobacilli against C. sakazakii ATCC 29544 investigating their possible mechanism of action by a flow cytometric protocol to detect the membrane integrity of bacterial cells.
Materials and methods
Bacterial strains and culture conditions
Five Lactobacillus spp., including Lactobacillus acidophilus ATCC 4356, Lactobacillus bulgaricus LBGR1, Lactobacillus casei rhamnosus ATCC 7469, Lactobacillus paracasei B21060 and Lactobacillus salivarius ATCC 11741, were included in this study. All the strains were grown on MRS agar (Oxoid, Milan, Italy) at 37 °C for 24–48 h under microaerophilic conditions (5% O2; 10% CO2, 85% N2).
The reference strain C. sakazakii ATCC 29544 was used in this study as an artificial contaminant microorganism. The strain was cultivated on Tryptone Soy Agar (TSA, Oxoid) at 37 °C for 24 h in aerobic conditions.
Time-kill studies
Time-kill studies were performed against C. sakazakii ATCC 29544 using the cell-free culture supernatants (CFCSs) of the different lactobacilli obtained as reported in Campana et al. (2012). Briefly, the strains were inoculated into 50 ml of MRS broth (Oxoid) and incubated at 37 °C for 18–24 h under microaerophilic conditions. At the end of incubation, each bacterial suspension was centrifuged at 17,000 rpm at 4 °C for 15 min; the supernatants (crude CFCSs) were adjusted to 6.5 with NaOH 5 M and collected filtered (0.22 µm pore size filters) (VWR, Milan, Italy) to remove any remaining bacteria. Aliquots (10 ml each) of crude CFCSs were freeze-dried to obtain a final concentration of 2.5 × (2.5 × CFCSs) and stored at − 20 °C. For time-kill experiments, 500 µl of overnight culture of Cronobacter sakazakii ATCC 29544, adjusted to 106 cfu/ml, was incubated at 37 °C with an equal volume of each crude or 2.5 × CFCS. At baseline and after 2, 4 and 8 h of incubation, aliquots were aseptically removed, serially diluted in physiological saline solution and spread in triplicate onto TSA (Oxoid). Plates were incubated at 37 °C for 24 h for cfu/ml determination.
Flow cytometry
To evaluate the membrane integrity of C. sakazakii ATCC 29544 cells after treatment with crude and 2.5 × CFCSs, flow cytometric (FCM) analysis was applied using SYBR Green-I and PI double staining. This combination of fluorochromes allows distinguishing 3 different bacterial populations: viable (SYBR Green positive cells), dead (PI positive cells) and damaged (SYBR Green and PI positive cells) cells.
C. sakazakii ATCC 29544 overnight suspension (500 µl) was treated with an equal volume of crude or 2.5 × CFCS for 30 min, 1 and 2 h and, then, labelled with SYBR Green-I (1/10.000, v/v) (Molecular Probes, Inc., Eugene, OR, USA) and PI (10 μg/ml) (Sigma) for 15 min in the dark at room temperature (Barbesti et al. 2000). Before being processed by FACSCalibur (Becton–Dickinson), equipped with 488 nm laser, 30 µl of CytoCount beads (DaKoCytomation) were added to each sample in order to obtain absolute cell numbers. Multi-parametric analyses were performed on both scattering signals (FSC and SSC) and FL1/FL3 channels. In particular, the SYBR Green I green fluorescence was detected on FL1 (530/30) while PI red fluorescence was detected on FL3 (> 670). The data were analysed using CellQuest software (Becton–Dickinson Biosciences).
Statistical analysis
Statistical analysis was performed using Prism 5.0 (GraphPad Software, Inc., La Jolla, USA). All the data are expressed as the mean values obtained in three independent experiments performed in duplicate. The conditions necessary to perform parametric tests were checked before conducting the analysis, otherwise non-parametric tests were utilized. The level of significance was considered α = 0.05.
Results and discussion
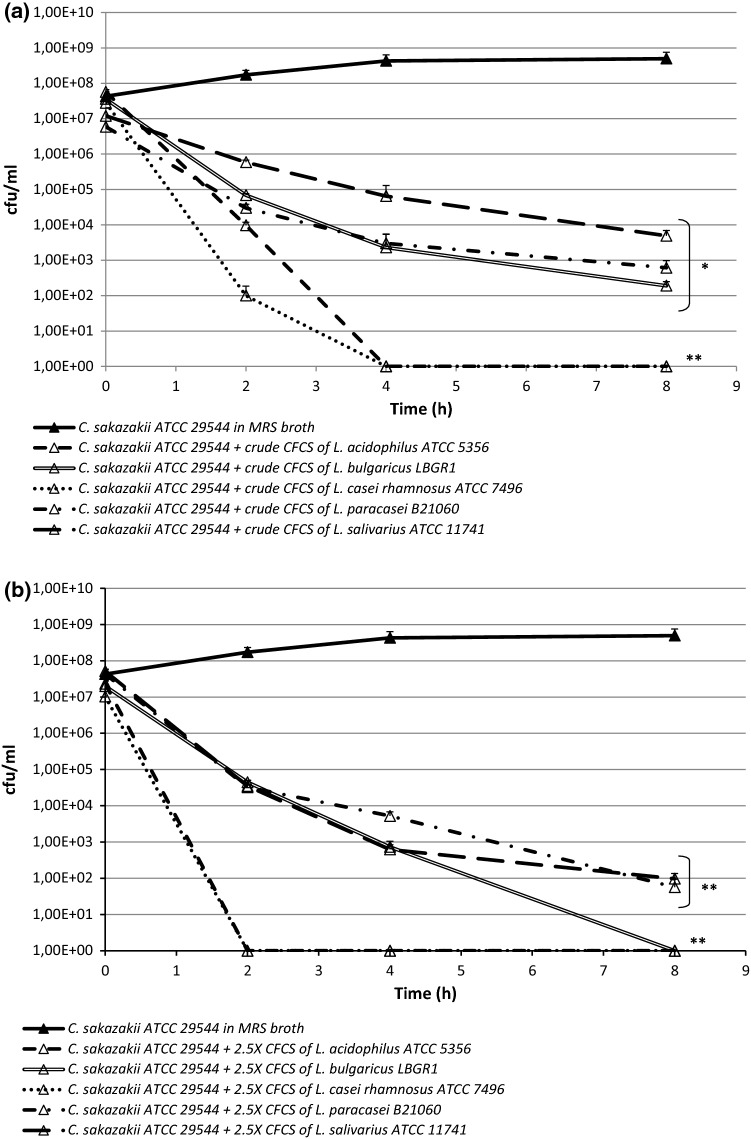
In the present study, the antimicrobial effect of CFCSs obtained from different lactobacilli was tested against C. sakazakii ATCC 29544. The crude CFCSs of L. casei rhamnosus ATCC 7469 and L. acidophilus ATCC 4356 completely inhibited the growth of C. sakazakii ATCC 29544 after 4 h of incubation (P < 0.01), while the others crude CFCSs showed an antimicrobial effect after 8 h of incubation, with cfu/ml values ranging from 4.09 × 103 to 1.9 × 102 cfu/ml in presence of L. salivarius ATCC 11741 or L. bulgaricus LBGR1 respectively (P < 0.05) (Fig. 1a). All the 2.5 × concentrated CFCSs showed a more remarkable effect on C. sakazakii ATCC 29544 growth ability in comparison to the related crude CFCSs, with a reduced time of exposition (P < 0.01). Indeed, the complete growth inhibition of C. sakazakii ATCC 29544 was reached after 2 h of incubation with L. casei rhamnosus ATCC 7469 and L. acidophilus ATCC 4356 2.5 × CFCSs. Moreover, 7.33 × 102 and 6.2 × 102 cfu/ml were evidenced after 4 h of incubation with 2.5 × CFCS of L. bulgaricus LBGR1 and L. salivarius ATCC 11741 respectively, reaching the complete growth inhibition of C. sakazakii ATCC 29544 in the case of 2.5 × CFCS of L. bulgaricus LBGR1 (Fig. 1b). Our results indicated that most of the tested lactobacilli effectively produced antimicrobial compounds able to limit the growth of C. sakazakii ATCC 29544. These data confirmed that Lactobacillus spp. showed to exert the greatest antimicrobial activity against C. sakazakii. Indeed, Awaisheh et al. (2013) reported the antibacterial activity of supernatants obtained from L. acidophilus and L. casei, isolated from feces of healthy infants, against different strains of C. sakazakii; similarly, Charchoghlyan et al. (2016) and Kim et al. (2018) indicated the ability of different Lactobacillus species to inhibit the growth of this pathogenic microorganism.
Fig. 1.
Effect of CFCSs obtained from different Lactobacillus spp. strains against C. sakazakii ATCC 29544 growth assessed by time-kill studies: a crude CFCSs; b 2.5 × concentrated CFCSs. All data were expressed as the mean of three independent experiments performed in duplicate. Asterisks represent values statistically significant (*P < 0.05; **P < 0.01) compared to the related untreated controls
In the last decade, new approaches for the rapid assessment of bacterial viability have been increasingly favored, and among these, the flow cytometry with different stains (fluorochromes) in combination has been shown to be a powerful tool for a rapid analysis of bacterial populations (Leonard et al. 2016). For this reason, we used FCM to verify the membrane integrity of C. sakazakii ATCC 29544 after exposure to the different CFCSs. The combination of fluorochromes allows distinguishing 3 different bacterial populations: viable (SYBR Green positive cells), dead (PI positive cells) and damaged (SYBR Green and PI positive cells) cells. In general, a decrease of viable cells with a parallel increase of dead cells was observed at all the examined time-points (30 min, 1 h and 2 h) (Table 1). In detail, after 30 min of exposition, the percentage of SYBR green I positive C. sakazakii ATCC 29544 cells ranged from 38% in presence of 2.5 × CFCS of L. casei rhamnosus ATCC 7469 to 87% with crude CFCS of L. salivarius ATCC 11741. Parallel, the higher percentages of dead cells (32%) and damaged cells (30%) were observed in presence of 2.5 × CFCS of L. casei rhamnosus ATCC 7469. After 1 h of exposition, the percentage of live cells drastically decreased to 7 and 5% in presence of L. casei rhamnosus ATCC 7469 crude and 2.5 × CFCSs respectively; similarly, only 3% of live C. sakazakii ATCC 29544 were detected in presence of 2.5 × CFCS of L. acidophilus ATCC 4356. A concomitant increase of dead and damaged C. sakazakii ATCC 29544 cells percentages were evidenced, with 74 and 81% of dead cells in presence of L. casei rhamnosus ATCC 7469 crude and 2.5 × CFCSs respectively. Similarly, 45 and 61% of dead cells were determined in presence of L. acidophilus ATCC 4356 crude and 2.5 × CFCSs respectively. The percentage of damaged C. sakazakii ATCC 29544 cells proportionally increased with all the others crude and 2.5 × CFCSs. After 2 h of exposure to CFCSs, in most cases a remarkable general decrease of C. sakazakii ATCC 29544 live cells was observed; L. acidophilus ATCC 4356 crude and 2.5 × CFCSs showed the highest antimicrobial effect with the complete loss of C. sakazakii ATCC 29544 viability (0 and 1% of live cells respectively). Analogously, low percentage of live C. sakazakii ATCC 29544 cells (1%) was detected in presence of 2.5 × CFCS of L. acidophilus ATCC 4356. From our data, can be observed that after a relatively short time of exposure to the different CFCSs (from 30 min to a maximum of 1 h), the membrane of C. sakazakii ATCC 29544 was progressively compromised up to the final death after 2 h of exposure to the crude and 2.5 × CFCSs of L. casei rhamnosus ATCC 7469 (83 and 96% of dead cells respectively). The observed bactericidal effect probably results from the production of such as organic acids, hydrogen peroxide, bacteriocins and carbon peroxide, representing one of the action mechanism of lactobacilli to contrast pathogens growth with a positive effect to human health (Lebeer et al. 2008). Indeed, the recent investigation of Yi et al. (2018) reported as the bacteriocin BMP11 produced by L. crustorum MN047 was able to destroy the integrity of C. sakazakii envelope, thus causing cell wall perforation and membrane permeabiliation. In this sense, we also have evidenced the ability of selected CFCSs (crude and 2.5 ×) to progressively inhibit the growth of C. sakazakii inducing changes in the membrane permeability with a gradual compromising of the treated cells. For these reasons, our results suggest that CFCSs could be directly used as an antimicrobial avoiding costly recovery processes since the exhausted broths containing mixtures of organic acids, thus providing new perspectives for the use of this “natural” antimicrobial compound. Nevertheless, toxicological studies must be performed to determine the true potential use of CFCSs for different fields of application, such in infant foods. In term of mechanism of action, FCM analyses showed that CFCSs altered the membrane permeability, as resulted by the coexistence of different subpopulations (viable, dead, and damaged) after antimicrobial treatment (Ciandrini et al. 2016; Díaz et al. 2010). Further studies are required to identify the metabolite components responsible of the observed antimicrobial activity and to obtain more information on their action mechanisms.
Table 1.
Effect of CFCSs on membrane permeability of C. sakazakii ATCC 29544
| Percentages (%, mean ± standard deviation) of bacterial cells | |||
|---|---|---|---|
| Damaged | Dead | Live | |
| 30 min | |||
| C. sakazakii ATCC 29544 plus | |||
| L. acidophilus ATCC 4356 crude CFCS | 23 (± 2.1) | 12 (± 0.5) | 65 (± 0.2) |
| L. acidophilus ATCC 4356 2.5 × CFCS | 31 (± 1.1) | 14 (± 0.2) | 55 (± 1.2) |
| L. bulgaricus LBGR1 crude CFCS | 15 (± 1.5) | 5 (± 0.3) | 80 (± 0.7) |
| L. bulgaricus LBGR1 2.5 × CFCS | 21 (± 0.5) | 8 (± 1.0) | 71 (± 2.0) |
| L. casei rhamnosus ATCC 7469 crude CFCS | 25 (± 1.1) | 20 (± 2.5) | 55 (± 0.7) |
| L. casei rhamnosus ATCC 7469 2.5 × CFCS | 30 (± 0.5) | 32 (± 1.5) | 38 (± 1.2) |
| L. paracasei B21060 crude CFCS | 19 (± 1.7) | 4 (± 0.5) | 77 (± 0.5) |
| L. paracasei B21060 2.5 × CFCS | 22 (± 2.5) | 4 (± 0.2) | 74 (± 0.3) |
| L. salivarius ATCC 11741 crude CFCS | 10 (± 1.4) | 3 (± 0.7) | 87 (± 0.5) |
| L. salivarius ATCC 11741 2.5 × CFCS | 15 (± 0.7) | 5 (± 0.5) | 80 (± 1.2) |
| C. sakazakii ATCC 29544 | 1 (± 0.3) | 2 (± 0.2) | 97 (± 0.5) |
| 1 h | |||
| C. sakazakii ATCC 29544 plus | |||
| L. acidophilus ATCC 4356 crude CFCS | 12 (± 0.5) | 45 (± 0.5) | 43 (± 0.2) |
| L. acidophilus ATCC 4356 2.5 × CFCS | 36 (± 0.5) | 61 (± 1.5) | 3 (± 0.1) |
| L. bulgaricus LBGR1 crude CFCS | 17 (± 0.8) | 11 (± 0.2) | 71 (± 0.5) |
| L. bulgaricus LBGR1 2.5 × CFCS | 39 (± 0.1) | 25 (± 0.5) | 36 (± 0.3) |
| L. casei rhamnosus ATCC 7469 crude CFCS | 19 (± 0.2) | 74 (± 0.4) | 7 (± 0.3) |
| L. casei rhamnosus ATCC 7469 2.5 × CFCS | 14 (± 0.5) | 81 (± 0.3) | 5 (± 0.5) |
| L. paracasei B21060 crude CFCS | 20 (± 0.4) | 10 (± 0.5) | 70 (± 1.5) |
| L. paracasei B21060 2.5 × CFCS | 17 (± 0.5) | 13 (± 0.7) | 70 (± 0.9) |
| L. salivarius ATCC 11741 crude CFCS | 10 (± 0.3) | 5 (± 0.5) | 85 (± 0.5) |
| L. salivarius ATCC 11741 2.5 × CFCS | 20 (± 0.2) | 12 (± 0.6) | 68 (± 0.7) |
| C. sakazakii ATCC 29544 | 2 (± 0.5) | 1 (± 0.2) | 97 (± 0.2) |
| 2 h | |||
| C. sakazakii ATCC 29544 plus | |||
| L. acidophilus ATCC 4356 crude CFCS | 10 (± 0.5) | 57 (± 0.3) | 33 (± 0.5) |
| L. acidophilus ATCC 4356 2.5 × CFCS | 18 (± 0.4) | 81 (± 0.5) | 1 (± 0.5) |
| L. bulgaricus LBGR1 crude CFCS | 20 (± 0.5) | 18 (± 0.2) | 62 (± 0.2) |
| L. bulgaricus LBGR1 2.5 × CFCS | 48 (± 0.7) | 21 (± 0.5) | 31 (± 0.2) |
| L. casei rhamnosus ATCC 7469 crude CFCS | 16 (± 1.2) | 83 (± 1.0) | 1 (± 0.5) |
| L. casei rhamnosus ATCC 7469 2.5 × CFCS | 4 (± 0.5) | 96 (± 0.4) | 0 |
| L. paracasei B21060 crude CFCS | 14 (± 0.4) | 22 (± 0.5) | 64 (± 1.0) |
| L. paracasei B21060 2.5 × CFCS | 20 (± 0.4) | 17 (± 0.5) | 63 (± 0.1) |
| L. salivarius ATCC 11741 crude CFCS | 10 (± 0.5) | 6 (± 0.3) | 84 (± 0.2) |
| L. salivarius ATCC 11741 2.5 × CFCS | 37 (± 0.1) | 14 (± 0.2) | 49 (± 0.5) |
| C. sakazakii ATCC 29544 | 2 (± 0.4) | 3 (± 0.5) | 95 (± 0.4) |
Data represent the percentages of damaged, dead and live cells after exposure for 30 min, 1 h and 2 h to several crude and 2.5 × CFCSs, as determined by FCM using the double staining SYBR Green I and PI
Authors’ contributions
WB conceive the study and RC designed the experiments; SF, EC and RC performed the experiments and analysed data; AM carried out FCM analysis; RC and WB wrote the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Awaisheh SS, Al-Nabulsi AA, Osaili TM, Ibrahim S, Holley R. Inhibition of Cronobacter sakazakii by heat labile bacteriocins produced by probiotic LAB isolated from healthy infants. J Food Sci. 2013;78:M1416–M1420. doi: 10.1111/1750-3841.12209. [DOI] [PubMed] [Google Scholar]
- Aymerich T, Picouet PA, Monfort JM. Decontamination technologies for meat products. Meat Sci. 2008;78:114–129. doi: 10.1016/j.meatsci.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barbesti S, Citterio S, Labra M, Baroni MD, Neri MG, Sgorbati S. Two and three-color fluorescence flow cytometric analysis of immune identified viable bacteria. Cytometry. 2000;40:214–218. doi: 10.1002/1097-0320(20000701)40:3<214::AID-CYTO6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Campana R, Federici S, Ciandrini E, Baffone W. Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr Microbiol. 2012;64:371–378. doi: 10.1007/s00284-012-0080-0. [DOI] [PubMed] [Google Scholar]
- Charchoghlyan H, Kwon H, Hwang DJ, Lee JS, Lee J, Kim M. Inhibition of Cronobacter sakazakii by Lactobacillus acidophilus n.v. Er2 317/402. Korean J Food Sci Anim Resour. 2016;36:635–640. doi: 10.5851/kosfa.2016.36.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciandrini E, Campana R, Casettari L, Perinelli DR, Fagioli L, Manti A, Palmieri GF, Papa S, Baffone W. Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl Microbiol Biotechnol. 2016;100:6767–6777. doi: 10.1007/s00253-016-7531-7. [DOI] [PubMed] [Google Scholar]
- Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs (Text with EEA relevance). http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1432490174116&uri=CELEX:32007R1441. Accessed 7 Dec 2007
- Díaz M, Herrero M, García LA, Quirós C. Application of flow cytometry to industrial microbial bioprocesses. Biochem Eng J. 2010;48:385–407. doi: 10.1016/j.bej.2009.07.013. [DOI] [Google Scholar]
- Hunter CJ, Bean JF. Cronobacter: an emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J Perinatol. 2013;33:581–585. doi: 10.1038/jp.2013.26. [DOI] [PubMed] [Google Scholar]
- Jason J. The roles of epidemiologists, laboratorians, and public health agencies in preventing invasive Cronobacter infection. Front Pediatr. 2015;3:110. doi: 10.3389/fped.2015.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyantanda G, Shumyak L, Archibald LK. Cronobacter species contamination of powdered infant formula and the implications for neonatal health. Front Pediatr. 2015;3:56. doi: 10.3389/fped.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jeong D, Song K, Kang I, Kim H, Seo K. Culture supernatant produced by Lactobacillus kefiri from kefir inhibits the growth of Cronobacter sakazakii. J Dairy Res. 2018;85(1):98–103. doi: 10.1017/S0022029917000802. [DOI] [PubMed] [Google Scholar]
- Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L, Bouarab Chibane L, Ouled Bouhedda B, Degraeve P, Oulaha N. Recent advances on multi-parameter flow cytometry to characterize antimicrobial treatments. Front Microbiol. 2016;7:1225. doi: 10.3389/fmicb.2016.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ge W, Li K, Gan J, Zhang Y, Luo R, Chen L, Liang Y, Wang Q, Xi M, Xia X, Wang X, Yang B. Prevalence and characterisation of Cronobacter sakazakii in retail milk-based infant and baby foods in Shaanxi, China. Foodborne Pathog Dis. 2016;13:221–227. doi: 10.1089/fpd.2015.2074. [DOI] [PubMed] [Google Scholar]
- Lin TH, Pan TM. Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J Microbiol Immunol Infect. 2019;52(3):409–417. doi: 10.1016/j.jmii.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Lucera A, Costa C, Conte A, Del Nobile MA. Food applications of natural antimicrobial compounds. Front Microbiol. 2012;3:287. doi: 10.3389/fmicb.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S, Nybom S, Meriluoto J, Collado MC, Vesterlund S, El-Nezami H. Interaction of probiotics and pathogens: benefits to human health? Curr Opin Biotechnol. 2010;21:157–167. doi: 10.1016/j.copbio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Teixeira AA, de Paula RA, Mantovani HC. (Awaisheh, Al-Nabulsi et al. 2013) Food Microbiol. 2006;23:213–219. doi: 10.1016/j.fm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Yi L, Li X, Luo L, Lu Y, Yan H, Qiao Z, Lü X. A novel bacteriocin BMP11 and its antibacterial mechanism on cell envelope of Listeria monocytogenes and Cronobacter sakazakii. Food Control. 2018;91:160–169. doi: 10.1016/j.foodcont.2018.03.038. [DOI] [Google Scholar]