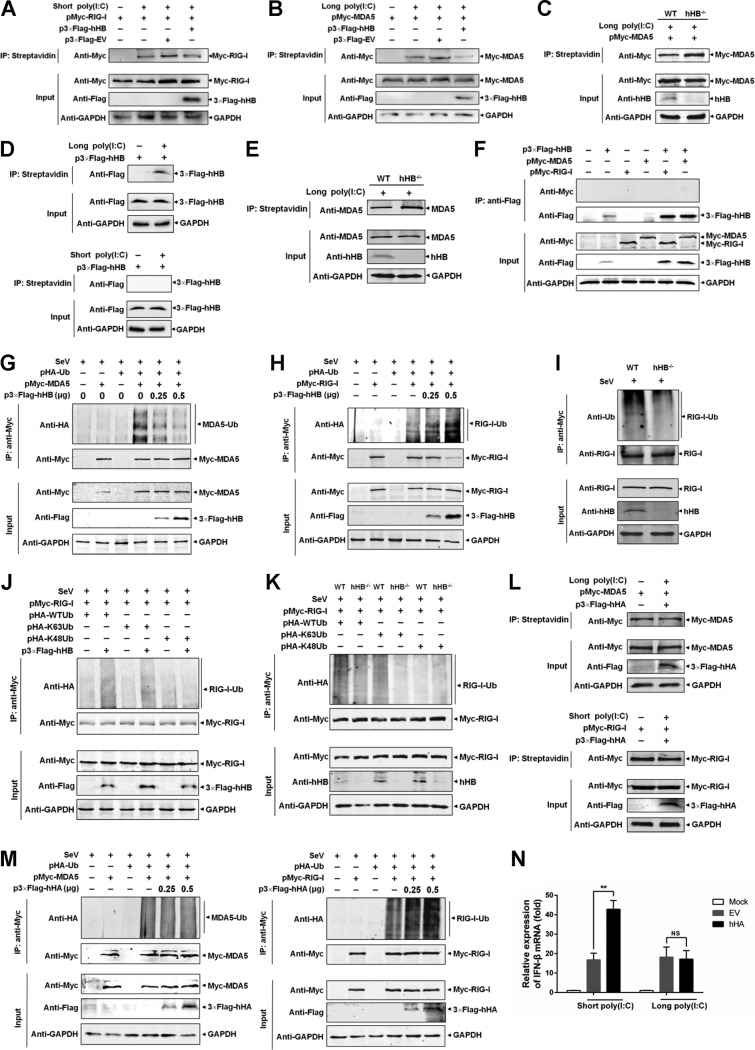
FIG 6.
hHB inhibits the binding of dsRNA to MDA5 but not to RIG-I and enhances the ubiquitination of RIG-I. (A) hHB has no influence on the interaction of exogenous RIG-I with short poly(I·C). HEK293T cells were cotransfected with pMyc-RIG-I and p3×Flag-hHB for 48 h. Then the cells were lysed, and the cell lysates were incubated with the photobiotin-labeled short poly(I·C) for 4 h at 4°C. Then the biotinylated RNA-protein compounds were immunoprecipitated (IP) with Dynabeads Streptavidin for 1 h at room temperature. The bound Myc–RIG-I proteins were applied to SDS-PAGE gels and then analyzed by Western blotting. (B and C) hHB inhibits the binding of exogenous MDA5 to the long poly(I·C). HEK293T cells were transfected with pMyc-MDA5 and p3×Flag-hHB (B), or the hHB−/− and the WT cells were transfected with pMyc-MDA5 (C). The cell lysates were collected at 48 hpt and incubated with the photobiotin-labeled long poly(I·C) for 4 h at 4°C. Then the RNA-bound proteins were precipitated and analyzed. (D) hHB inhibits the binding of endogenous MDA5 to the long poly(I·C). The lysates of the hHB−/− and the WT cells were incubated with the photobiotin-labeled long poly(I·C) as described above. Then the long-poly(I·C)-bound proteins were precipitated and analyzed. (E) hHB interacted with long poly(I·C) but not short poly(I·C). HEK293T cells were transfected with p3×Flag-hHB. At 48 hpt, the cell lysates were collected, and the long-poly(I·C)-bound proteins were precipitated and analyzed as described above. (F) Co-IP analysis of interaction between hHB and RIG-I or MDA5 as described in Materials and Methods. (G and H) The effects of hHB on the ubiquitination of exogenous RIG-I and MDA5. HEK293T cells were cotransfected with the indicated plasmids for 12 h and then infected with SeV for 12 h. The prepared cell extracts were analyzed by IP analysis using an anti-Myc monoclonal antibody. (I) The endogenous ubiquitination of RIG-I in hHB−/− cells. The hHB−/− and WT cells were infected with SeV for 12 h. The prepared cell extracts were analyzed by IP analysis using an anti-RIG-I monoclonal antibody. (J and K) The effects of hHB on the K63-linked ubiquitination of RIG-I and MDA5. HEK293T cells or hHB−/− cells were cotransfected with the indicated plasmids for 12 h and then infected with SeV for 12 h. The prepared cell extracts were analyzed by IP analysis using an anti-Myc monoclonal antibody. (L) hHA has no influence on the interactions of RIG-I–short poly(I·C) and MDA5–long poly(I·C). HEK293T cells were cotransfected with pMyc-RIG-I or pMyc-MDA5 and p3×Flag-hHA for 48 h. Then the cells were lysed, and the short-poly(I·C)- or long-poly(I·C)-bound proteins were precipitated and analyzed as described above. (M) The effect of hHA on the ubiquitination of RIG-I and MDA5. HEK293T cells were cotransfected with the indicated plasmids for 12 h and then infected with SeV for 12 h. The prepared cell extracts were analyzed by IP analysis using an anti-Myc monoclonal antibody. (N) Overexpression of hHA promoted the RIG-I-mediated activation of IFN-β transcription but not the MDA5-mediated activation of IFN-β transcription. HEK293T cells were transfected with p3×Flag-EV or p3×Flag-hHA and short poly(I·C) or long poly(I·C) for 24 h, and the IFN-β mRNA level in cells was analyzed. The data represent the means ± standard deviations from three independent experiments. Significant differences are denoted as follows: **, P < 0.01; NS, not significant (P > 0.05). Ub, ubiquitin; HA, hemagglutinin.