Figure 1.
ATP-Dependent Head Engagement (E) State of Smc Head Domains
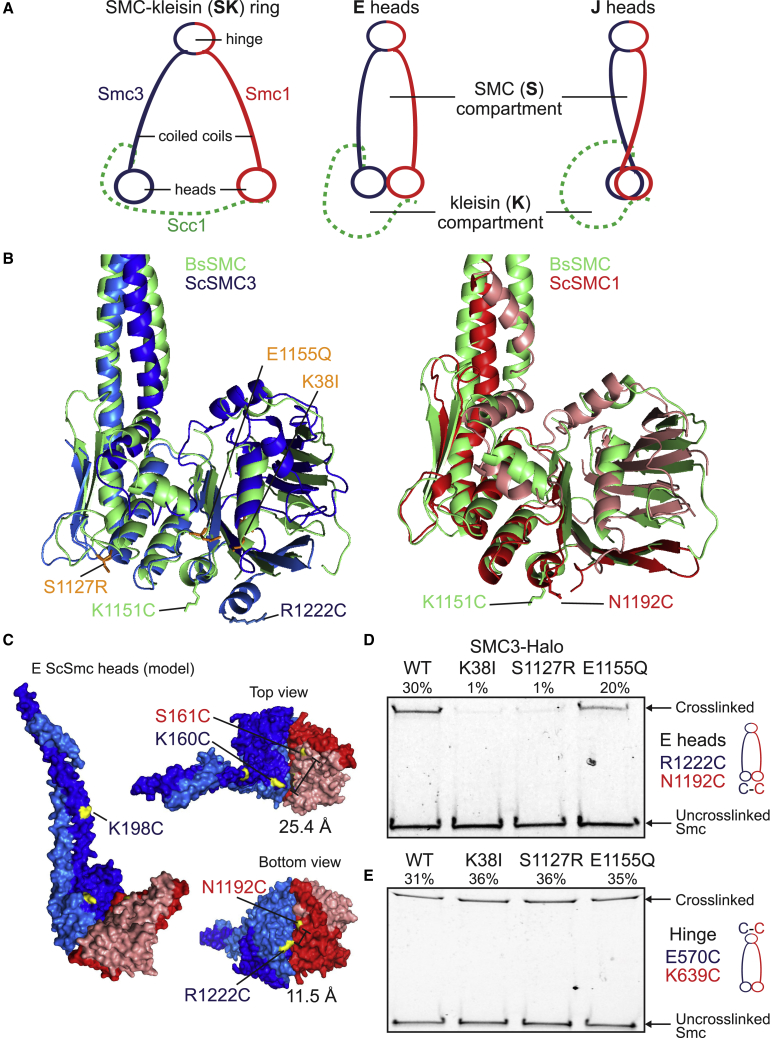
(A) Schematic representation of the cohesin compartments.
(B) Structure alignment of Sc Smc3 head (PDB: 4UX3, blue) and Sc Smc1 head (PDB: 1W1W, red) to Bs Smc head (PDB: 3ZGX, green). Selected residues displaying efficient cross-linking when mutated to cysteine are marked (for B. subtilis, see Diebold-Durand et al., 2017). Residues associated with the Smc3 ATP binding mutant (K38I), the signature motif mutant (S1127R), and the ATP hydrolysis mutant (E1155Q) are displayed in orange.
(C) Model of ATP-engaged Smc3/Smc1 heads in surface representation in front (left) and top and bottom views (right). ATP-engaged head dimer is constructed by superimposition of Smc1 head to one of Smc3 head homodimer (Gligoris et al., 2014). Distance between selected residues is given.
(D and E) In vivo cysteine cross-linking of Smc1 proteins with Halo-tagged wild-type and ATPase mutant Smc3. Cross-linking of Smc1(N1192C) and Smc3(R1222C) E head residues (C) or Smc1(K639C) and Smc3(E570C) hinge residues (D) was performed in vivo using BMOE. Cell extracts were labeled with HaloTag-TMR ligand. Smc-HaloTag species were separated by SDS-PAGE and quantified by in-gel fluorescence. Percentage of cross-link efficiency is indicated.
See also Figure S1.