Figure 3.
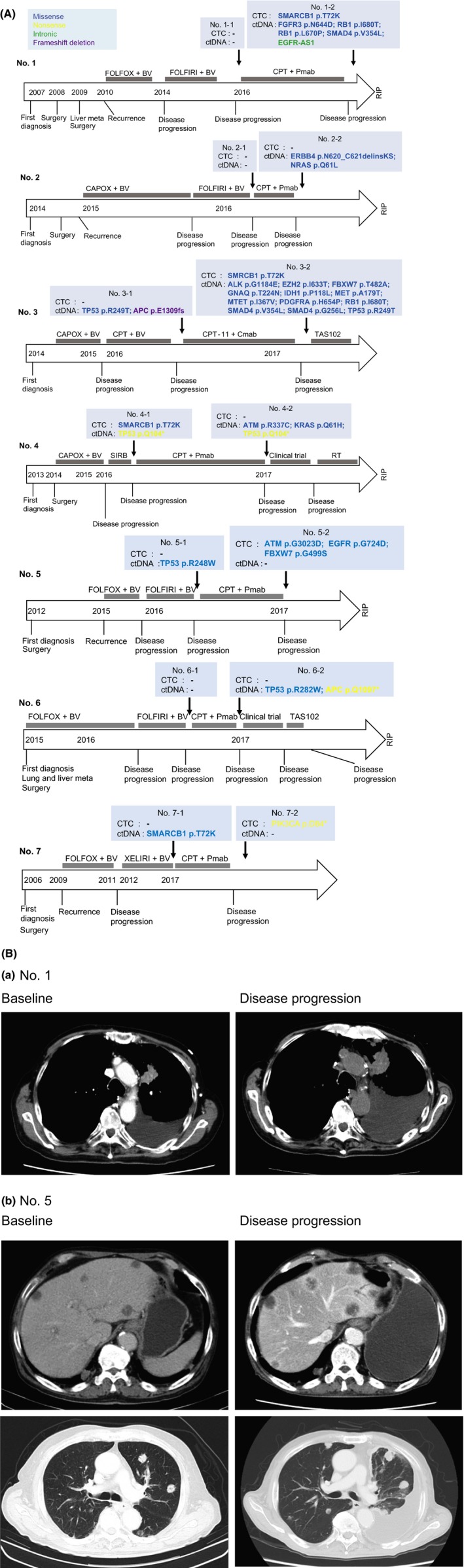
A, Clinical course in colorectal cancer patients who received anti–epidermal growth factor receptor (EGFR) therapy and genomic alterations in circulating tumor cells (CTC) and ctDNA. A, Monitoring genomic profiles of CTC and ctDNA during anti–EGFR therapy.BV, bevacizumab; CAPOX, capecitabine, oxaliplatin; Cmab, cetuximab; CPT, irinotecan; FOLFIRI, folinic acid, fluorouracil and irinotecan; FOLFOX, folinic acid, fluorouracil and oxaliplatin; Pmab, panitumumab; RT, radiation therapy; SIRB, tegafur/gimeracil/oteracil, irinotecan and bevacizumab; TAS102, trifluridine and tipiracil. B, Representative CT images. (a) Growth of lung metastases and increased pleural effusion were observed during disease progression in patient 1. (b) Growth of liver and lung metastases and increased pleural effusion were observed during disease progression in patient 5
