Abstract
Aims
Myocardial infarction (MI) and heart failure (HF) are risk factors for the development of depression, additionally worsening the quality of life and patient outcome. How HF causes depression and how depression promotes HF remain mechanistically unclear, which is at least partly caused by the difficulty of in vivo modelling of psychosomatic co‐morbidity. We aimed to study the potential sequence of events with respect to different depression aspects upon HF.
Methods and results
Male C57BL6 mice underwent MI, followed by behavioural and echocardiographic characterization. Motility, exploration, and anxiety‐like behaviour were unaffected in mice after MI. We did not observe increased depressive‐like behaviour in the sucrose preference, tail suspension, or Porsolt forced swim test. Mice did not display signs of learned helplessness (LH) when compared to sham. Accordingly, cluster analysis revealed only a slightly higher quota of LH in HF (38%) vs. sham mice (32%). But strikingly, three‐group cluster analysis revealed an additional intermediate subpopulation at risk for LH after HF (29%). Interestingly, this population featured elevated cardiac expression of nr4a1.
Conclusions
The LH paradigm uncovered a subtle predisposition to depressive‐like behaviour after MI, whereas testing for anhedonia and despair was insufficient to show a behavioural shift in mice. Therefore, we suggest an accumulating risk profile and a multiple‐hits hypothesis regarding the pathogenesis of co‐morbid depression after MI. Symptoms of LH may present a marker of subclinical depression after MI, the impact of which remains to be investigated. The proposed sequence of behavioural testing enables the mechanistic dissection of cardio‐psychogenic signalling in the future.
Keywords: Myocardial infarction, Heart failure, Depression, Anxiety, Learned helplessness, Co‐morbidity
Introduction
Heart failure (HF) is the leading cause of death in Europe and depression the major cause of disability worldwide.1 The prevalence of major depression is up to five times higher in HF patients when compared with the general population.2 Conversely, patients with symptoms of depression are at increased risk of HF.3 Taken together, a reciprocal hazard model of depression and HF is suggested. Even though myocardial infarction (MI) and ischaemic heart disease present the leading cause of HF in Europe1 and an important risk factor for the subsequent development of depression,4 the pathogenesis of increased vulnerability to depression and anxiety in response to MI remains unclear.4 Patients with HF and co‐morbid depression show poorer clinical outcome, including elevated morbidity, rate of hospitalization, and mortality.5, 6 Pathogenetically oriented treatment options remain scarce. Psychotherapy and antidepressant medication have not shown beneficial impact on patient outcome so far.7, 8, 9, 10
This may be explained by a separate type of depression in the setting of HF, as opposed to spontaneous occurring depression in otherwise healthy individuals. Therefore, a basic mechanistic understanding remains necessary to develop novel therapeutic approaches. Unfortunately, the pursuit of suitable animal models to investigate the brain–heart axis in vivo remains a major challenge. And even though there is a reproducible rat model of good validity,11 a murine model of depression after MI remains missing. Several studies utilize a model of depression together with MI12, 13 to investigate the effects of depression on cardiac remodelling, but few publications focus on the pathogenesis of depression due to MI per se. In this regard, murine anhedonia over the course of 8 weeks after MI associated with expression changes of the orphan nuclear receptor 4a1 (nr4a1) has been observed.14
In behavioural research, tests of individual symptoms of depression, e.g. the sucrose preference test (anhedonia), the Porsolt forced swim test, and the tail suspension test (despair), have to be distinguished from models that induce depressive‐like behaviour.15 Although behavioural tests may unravel a distinct symptomology, the introduction of an animal model of depression at this point presents an opportunity to uncover an underlying disposition to depressive‐like behaviour. The learned helplessness (LH) paradigm presents a model of stress‐induced depression due to repetitive aversive events (mild electric foot shocks) that was first conceptualized by M. Seligman with translation into a clinically relevant mouse model by Chourbaji et al.16 Helplessness‐related coping deficits present a core symptom of human depression, and LH presents a clinically relevant model of depressive‐like behaviour in rodents with high validity.15, 17
To evaluate the pathogenesis of depression and anxiety in HF, MI or sham operation was performed in male C57BL6 mice with consecutive behavioural testing 3–4 weeks later. Finally, we aimed to uncover underlying behavioural tendencies by subjecting mice to the LH paradigm with consecutive cardiac characterization.
Methods
Experimental animals
Animal experiments were performed according to institutional and governmental guidelines. The study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 1985) and was approved by the authorities of the Regierungspräsidium Karlsruhe, Germany (G‐134/16). Every effort was made to minimize the number of animals used and their suffering. Animals were held with access to food and water ad libitum at a 12 h day‐and‐night‐rhythm at 21°C and 50–60% humidity. All behavioural testing was conducted during the active phase by the same experienced investigator that was blinded regarding the animals group affiliation.
Experimental design
Ten‐week‐old male C57BL6 mice were subjected to MI or sham operation 1 day after baseline echocardiography. After 24 h, blood was drawn to quantify infarct size via high‐sensitive Troponin T levels as previously described.18 Behavioural testing started at week 3 after MI and ended with the LH paradigm with subsequent follow‐up echocardiography and sacrifice by decapitation (Figure 1 ). Trunk blood was collected and spun at 14 000 g for 20 min at 4°C, and supernatants were stored at −80°C until further analysis. The heart was harvested, and left and right ventricles were dissected and immediately stored on liquid nitrogen. The brain was harvested and dissected into prefrontal cortex, hippocampus, and brain stem. Tissue was pulverized by a mortar and stored at −80°C until further evaluation.
Figure 1.

Schematic representation of experimental design. One day after baseline echocardiography, myocardial infarction (MI) or sham operation was performed with a subsequent period of rest for 2 weeks. In the third week, behavioural testing was started with the open field test, followed by the dark–light box test. Afterwards, pain sensitivity was tested via the hotplate test with consecutive testing for depressive‐like behaviour in the Porsolt forced swim, the tail suspension, and the sucrose preference tests. The learned helplessness paradigm was conducted with 2 days of consecutive conditioning and testing for helplessness on the third day. One day later, follow‐up echocardiography was performed with subsequent sacrifice of experimental animals.
Myocardial infarction and sham operation
We used the model of left anterior descending coronary artery ligation,19 to induce MI. Briefly, mice were injected with buprenorphine 0.1 mg/kg bw s.c. 30 min before anaesthetized with isoflurane (3 vol%), intubated, and put on a small animal ventilator. After thoracotomy, MI was induced by proximal ligation of the left anterior descending coronary artery. In sham‐operated mice, the myocardium was punctured without ligation. The operation took about 20 min and was followed by postoperative analgesia with s.c. buprenorphine.
Plasma preparation, measurement of Troponin T
Retroorbital blood was taken from isoflurane anaesthetized mice using haematocrit capillaries 24 h after the operation. Whole blood was centrifuged at 14 000 g for 20 min at 4°C. Supernatants were stored until further analysis at −80°C. For quantification of infarct size, high‐sensitive Troponin T (hs‐TnT) was measured using an automated Cobas Troponin T hs STAT Elecsys (Roche) as described previously.18 Corticosterone was quantified by radio‐immunosorbent assay as described elsewhere.20
Echocardiography
Cardiac function was evaluated by 2D echocardiography at baseline and 4 weeks after MI or sham operation under isoflurane volatile mask narcosis (1–3 vol%) at a constant temperature of 38°C using a Visual Sonics Vevo® 2100 with an MX550D transducer by a blinded investigator. Mice were shaved, and left ventricular parasternal short‐axis views were obtained in M‐mode imaging at the papillary muscle level. Four consecutive beats were used for measurements of left ventricular end‐diastolic internal diameter, left ventricular end‐systolic internal diameter, and left ventricular ejection fraction (EF). Evaluation was conducted at 400–500 bpm to avoid the confounding effects of heart rate on cardiac function.
Open field test
Locomotor testing was conducted in a square, grey open field arena of 50 × 50 cm2 with 25 lx illumination from above. Mice were placed individually into the arena and tracked for 10 min by a digital camera from above. Evaluated parameters included mean distance to center (cm), total distance moved (cm), and mean speed (cm/s) and are indicators of anxiety/exploration and locomotion. Tracking and quantification were performed with Limelight 4.1 (Actimetrics). After each animal, the arena was cleaned by 70% ethanol.
Dark–light box test
The dark–light box consists of a black 20 × 15 cm2 and a bright grey 30 × 15 cm2 compartment connected by an open door. The black compartment is covered by a lid from above, whereas the adjacent grey box is illuminated brightly from above by 600 lx. Mice were initially placed into the dark compartment to quantify latency to first exit, number of exits, and total time exploring. Tracking and evaluation were performed with Limelight 4.1 (Actimetrics) for 5 min. After each animal, the arena was cleaned by 70% ethanol.
Sucrose preference test
Mice were given simultaneous access to a bottle of 1% saccharose solution and one bottle of tap water for 48 h. After 24 h, the bottles were switched to exclude side‐preference impact. The fraction of sucrose solution consumed relative to total liquid intake was calculated according to the weight difference of corresponding bottles.
Hotplate test
To prevent altered pain sensitivity from influencing the parameters of LH, mice were subjected to the hotplate test. Individual mice were placed on a heated surface (Panlab model LE7406, Harvard Apparatus), maintained at 53°C (±0.3°C) for a maximum of 45 s. The time taken for the animals to withdraw the paw from the hotplate or jump was recorded with subsequent test termination.
Porsolt forced swim test
Mice were placed individually into a glass cylinder (23 cm height and 17 cm diameter), filled with water at 21°C up to a height of 12 cm and monitored for 6 min by a digital camera from the side. The first 2 min were accounted for as an acclimatization period with evaluation of the latter 4 min. Immobility was defined as floating with only movements, necessary to stay above water. Struggling/swimming was defined as time spent in active movement. Immobility was evaluated with FreezeFrame 4.0 (Actimetrics). The water was switched after each animal. Afterwards, mice were placed in front of a heating lamp to support quick recovery of body temperature.
Tail suspension test
Experimental animals were isolated and suspended 25 cm above the floor by the tip of the tail with tracking of immobility by a digital camera from the side for 6 min. The first 2 min were accounted for as an acclimatization period with evaluation of the latter 4 min. Mice were considered immobile only when they hung completely motionless. Evaluation was conducted with FreezeFrame 4.0 (Actimetrics).
Learned helplessness paradigm
In order to induce a depressive‐like state in experimental animals after testing of depressive‐like behaviour after MI or sham operation, a customized version of the murine model of LH as initially described by Chourbaji et al.16 was applied for reasons of animal welfare (refinement). On two consecutive days, mice were exposed to 240 inescapable foot shocks at 0.15 mA with a random duration of 1–3 s and randomly assigned inter‐trial intervals of 1–15 s for a total daily duration of 52 min in a shock box (Coulborn Instruments, Germany). On the third day, LH was evaluated by 30 shocks within a shuttle box, consisting of two 18 × 18 × 30 cm3 compartments, separated by a small gate. Foot shocks were announced by light 5 s before electric foot shock. Infrared‐light beams monitored shuttling between the compartments, including behavioural responses to light or shock. Experimental animals were capable to abolish the electric shocks beforehand in response to appearance of the light or terminate a triggered shock by switching the compartment. For data acquisition and assessment, Graphic State software (Coulborn Instruments) was used. In accordance with Churbaji et al.,16 the following behavioural reactions were defined: avoidance—adequate reaction to the light stimulus by switching compartments, escape—shuttling to the other compartment in response to an electric shock, and failure—lack of an attempt to escape. Moreover, escape latency was quantified as a parameter of helplessness. In addition, general activity was evaluated by quantification of the number of shuttles before the first foot shock (initial activity) and the number of compartment switches in between foot shocks (inter‐trial activity). Testing took place with a duration of 20–24 min with the duration depending on the experimental animal's ability to learn and respond accordingly. After each animal, the apparatus was cleaned with 70% ethanol. All mice, included in this study, received electric foot shocks because only about 30% of the shocked mice develop helplessness.16
RNA extraction and quantitative PCR
RNA was isolated from homogenized left ventricular, frontal cortex, hippocampal, and brain stem tissue using TRIzol (Invitrogen). Total RNA was digested with DNase, and cDNA synthesis of 500 ng of RNA was carried out by using a SuperScript first‐strand synthesis system for RT‐PCR (Invitrogen). Quantitative real‐time PCR was performed with Universal ProbeLibrary (Roche) by using TaqMan Universal PCR Mastermix (Applied Biosystems) and detection on a 7500 Fast Cycler (Applied Biosystems).
Statistical analysis
Results are expressed as mean ± SEM. Normal distribution was verified by the Kolmogorov–Smirnov test. Statistical analysis included one‐way ANOVA or Kruskal–Wallis test followed by Bonferroni or Dunn's post hoc test, respectively. Student's t‐test or Mann–Whitney U test was used when appropriate (PRISM Software, GraphPad). Two‐group cluster analysis was conducted according to Chourbaji et al.16 Three‐group cluster analysis was conducted using SPSS according to the parameters of LH (avoidances, escapes, escape latency, and escape failures) (SPSS, IBM). A P < 0.05 was considered statistically significant.
Results
Myocardial infarction‐induced heart failure is not a murine model of depressive‐like behaviour
Myocardial infarction significantly increased hs‐TnT plasma levels after 24 h (Figure 2 A), indicating a comparable initial cardiac damage among the groups. Four weeks later, EF was markedly reduced (Figure 2 B), and heart weight/body weight ratio elevated (Figure 2 C) as compared with sham controls. We tested for symptoms of depression with the sucrose preference test (anhedonia) (Figure 2 D), the Porsolt forced swim test (Figure 2 E), and the tail suspension test (Figure 2 F) (despair). All tests failed to reveal depressive‐like behaviour after MI when compared with sham. In summary, MI‐induced HF did not have a significant impact on depressive‐like behaviour with respect to anhedonia and despair in mice.
Figure 2.
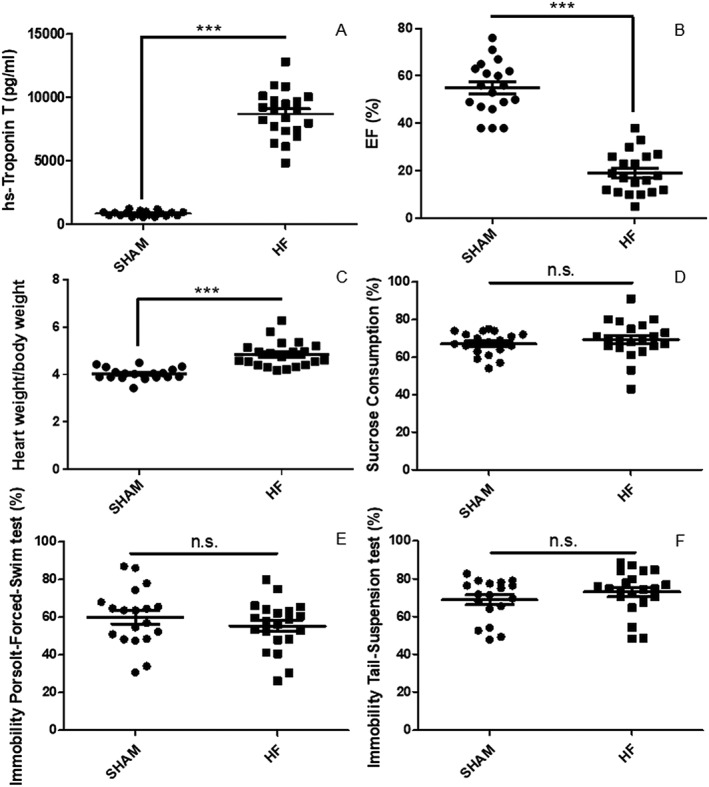
Heart failure (HF) following myocardial infarction (MI) is not a murine model of depressive‐like behaviour. MI induced a significant up‐regulation of high‐sensitive Troponin T (hs‐Troponin T) in plasma after 24 h when compared with sham‐operated animals (SHAM) (A). Left ventricular ejection fraction (EF) was significantly blunted 4 weeks after MI in HF mice (B), in line with the finding of a markedly up‐regulated heart weight/body weight ratio in HF mice (C). With respect to depressive‐like behaviour, we conducted testing of anhedonia via the sucrose preference test (D) and of despair, using the Porsolt forced swim test (E) and the tail suspension test (F). All three tests failed to reveal a significant increase in depressive‐like behaviour after MI. Data are presented as mean ± SEM. ***P < 0.0001 by Student's t‐test; n.s., not significant, n = 18–21 per group.
Myocardial infarction‐induced heart failure does not affect mobility, speed, or exploration
To evaluate mobility, speed, and exploration, we conducted the open field test (Figure 3 A–D). We did not observe an impact of HF after MI on total distance travelled (cm) (Figure 3 A), mean speed (cm/s) (Figure 3 B), mean distance to center (cm) (Figure 3 C), or time in center (%) (Figure 3 D). Taken together, we found mobility, speed, and exploratory behaviour to be unaffected by HF after MI.
Figure 3.

Heart failure (HF) after myocardial infarction does not affect motility, exploration, or anxiety‐like behaviour in mice. We observed no significant impact of HF regarding total distance travelled (A), mean speed (B), mean distance to center (C), and time in center (D) in the open field test. Furthermore, dark–light box testing (E–G) revealed no marked differences with respect to the total number of exits (E), the total amount of time exploring (F), or the latency to first exit (G). Data are presented as mean ± SEM; n.s., not significant by Student's t‐test (A, B, E–G) or Mann–Whitney U test (C) due to absence of normality, n = 12–21 per group.
Myocardial infarction‐induced heart failure does not induce anxiety‐like behaviour
In addition to open field testing, we conducted the dark–light box test (Figure 3 E–G) to further evaluate anxiety‐like behaviour. We did not observe differences between HF and sham mice with respect to the total number of exits (Figure 3 E), the total amount of time exploring (s) (Figure 3 F, dark–light box), or latency to first exit (s) (Figure 3 G, dark–light box).
Myocardial infarction‐induced heart failure does not predispose to learned helplessness
To evaluate the vulnerability to despair, we utilized the LH paradigm. After electric foot shocks on two consecutive days, all mice were tested for helplessness on the third day. General activity was evaluated by initial shuttling between the compartments of the shuttle box during the first 2 min before testing started and by shuttling between the compartments between the foot shocks. Here, we observed no significant differences between groups, suggesting comparable general activity in the MI and sham‐operated mice. Also, the amount of shock avoidances (Figure 4 A), escapes (Figure 4 B), mean escape latency (Figure 4 C), and escape failures (Figure 4 D) revealed no marked differences between HF and sham mice. Cluster analysis revealed 32% of sham animals to be helpless as opposed to 38% of HF mice (Figure 4E ). Previous testing for foot pain sensitivity did not reveal any marked differences between groups (Figure 4 F). In summary, we did not observe significant helplessness due to HF after MI.
Figure 4.

Heart failure (HF) after myocardial infarction does not enhance learned helplessness (LH) per se. To evaluate a predisposition to despair, the learned helplessness paradigm was applied. The amount of shock avoidances (A), escapes (B), mean escape latency (C), and escape failures (D) revealed no marked differences between mice with HF or after sham operation (SHAM). Cluster analysis showed 32% of SHAM animals to be helpless as opposed to 38% of HF mice (E). Previous testing for foot pain sensitivity did not reveal any marked differences between groups (F). Data are presented as mean ± SEM; n.s., not significant by Student's t‐test, n = 19–21 per group.
Mice suffering from myocardial infarction‐induced heart failure contain a subpopulation at risk for learned helplessness
Because of behavioural inhomogeneity in the HF group, we conducted an additional three‐group cluster analysis with LH parameters: escapes (Figure 5 B), escape failures (Figure 5 C), escape latency (Figure 5 D), and avoidances (Figure 5 E). Thereby, a large intermediate subpopulation (IM) at risk for depressive‐like behaviour was revealed within the HF (29%) and to a lesser extent, the sham (11%) group (Figure 5 A). This IM group featured elevated left ventricular nr4a1 mRNA expression (Figure 5 F). In summary, we observed a population at risk for depressive‐like behaviour after MI in the HF group with up‐regulated cardiac nr4a1 expression.
Figure 5.

Mice with heart failure (HF) after myocardial infarction contain a subpopulation at risk for learned helplessness (LH) with distinguished cardiac expression of nr4a1. Advanced cluster analysis reveals a large intermediate (IM) subpopulation, based on LH parameters, suggestive of an accumulating risk profile for depression in HF after myocardial infarction (A). This is mirrored by the number of escapes (B), the amount of escape failures (C), mean escape latency (D), and avoidances (E). Interestingly, this group features elevated left ventricular (LV) nuclear receptor 4a1 (nr4a1) expression when compared with RES and LH mice (H). Data are presented as mean ± SEM, *P < 0.05 by ANOVA (Bonferroni), n = 5–15 per group.
Discussion
Clinically, the association of HF with co‐morbid depression and anxiety6, 21 as well as its impact on patient outcome is a well‐known phenomenon.22, 23, 24 However, the pathogenesis of increased vulnerability to depression and anxiety in HF remains largely unclear.4 Here, we aimed to investigate the behavioural effects of HF upon MI in mice. In our study, mice suffering from HF after MI did not display increased severity of depressive‐like or anxiety‐like symptoms when compared with sham‐operated mice. However, using the LH paradigm to unmask an underlying risk for depressive‐like behaviour, cluster analysis revealed a vulnerable subgroup. Therefore, we propose that MI‐induced HF is a risk factor of depressive‐like behaviour but is not suitable as a mouse model of depression or anxiety. The subtle shift towards a ‘more depressed’ phenotype may facilitate the development of depression after further aversive events (i.e. accumulating hits). Moreover, we observed increased cardiac nr4a1 mRNA expression in this critical intermediate subgroup, which has been shown to be both cardioprotective after MI by limiting inflammation25 and detrimental in chronic pressure‐induced HF by activating the hexosamine biosynthetic pathway.26 It will be interesting to investigate the role of cardiac nr4a1 signalling regarding cardio‐psychogenic crosstalk.
In conclusion, we found MI‐induced HF not to lead to the development of depressive‐like or anxiety‐like behaviour per se but to facilitate a subtle behavioural shift towards a more depressive phenotype. Therefore, we suggest a multiple‐hits hypothesis with accumulating risk after MI, potentially priming the heart–brain axis towards detrimental psychological and cardiovascular consequences in the future (Figure 6 ).
Figure 6.

Proposed model of subclinical and multiple‐hit‐induced depression upon heart failure after myocardial infarction. Myocardial infarction with subsequent heart failure may facilitate subclinical depression. Multiple hits of subjectively perceived uncontrollable stress lead to an accumulating risk profile—with the potential of distinct myocardial signalling—ultimately translating to clinical depression.
Myocardial infarction‐induced heart failure is not a murine model of depression or anxiety
We observed motility, exploration, and depressive‐like behaviour to be unaffected in mice with HF upon MI, which was also described by Wang et al.27 In contrast, Frey et al. reported a reduction of motility and exploration with increased anhedonia in HF mice.14 Discrepancies here may be based on shorter duration of HF in our study (3 vs. 6 weeks) as well as a shorter testing interval for anhedonia (48 h vs. 8 weeks), because the other study reported an accumulated reduction not fixed to a single time point. Also, Wann et al. published a model of depressive‐like anhedonia (sucrose preference test) and despair (Porsolt forced swim test) 2 weeks after MI in rats, which has been utilized for several follow‐up studies focusing on central nervous system apoptosis and inflammation.11, 28 The availability of a rat model of depressive‐like behaviour after MI may be due to timing/kinetics and species. Considering clinical and experimental data, the idea of MI as a murine model of depression remains suggestive.
Paradoxically, the prevalence of depression has been reported higher in patients suffering from heart disease than in the setting of, e.g. diabetes or cancer,29 whereas experimental mouse models of diabetes30 as well as cancer31 increased depressive‐like behaviour in mice. This inconsistency together with the lack of impact of treatment on patient outcome may ultimately hint at cardiogenic depression as a separate disease entity. However, non‐ischaemic HF by transaortic constriction also induced depressive‐like anhedonia,32 and the literature or our results here do not consolidate a specific effect of ischaemic HF. Because MI as a murine model of cardiogenic depression would enable novel therapeutic approaches via knockout studies, further investigation is warranted.
Myocardial infarction‐induced heart failure facilitates an underlying risk for depressive‐like behaviour, distinguished by elevated cardiac nr4a1 expression
Models of depression have been used to investigate the effect of depression on HF after myocardial ischaemia in mice13 and rats.12 However, evidence regarding the impact of HF after MI on susceptibility to behavioural alterations prompted by a model of depression was missing. Here, we underpin the absence of depressive‐like behaviour after MI in mice by the lack of elevated susceptibility to LH, which has been established as a clinically relevant model of depression.16 In our study, all mice were subjected to inescapable foot shocks. About 30% of sham and MI mice developed LH, which is in line with the literature discussed earlier. However, we utilized a slightly modified protocol for reasons of animal welfare (refinement) based on 240, as opposed to 360 foot shocks at 0.15 mA during conditioning. We cannot exclude that a more aggressive protocol of LH, e.g. utilizing foot shocks at 0.3 mA, might have shown different results. However, because we did not observe an increase in severity or incidence of depressive‐like behaviour, we suggest lower EF after MI as a risk factor, not sufficient by itself for the development of depressive‐like behaviour, proposing a multiple‐hits hypothesis of depression after MI (Figure 6 ).
The finding of an IM behavioural cluster after HF is indicative of a larger population at risk for the development of LH after MI when compared with sham. Because this group featured significantly up‐regulated cardiac nr4a1‐signalling, which has been shown to balance the myocardial inflammatory response after MI,25 one might speculate on the impact on cardio‐neurogenic signalling. This is particularly interesting because depression has also been described as a neuroinflammatory disease entity.33 However, so far, a divergent role of nr4a1 is reported in cardiac disease: Lehmann et al. recently elaborated a novel mechanism of nr4a1‐facilitated impact on protein glycosylation with subsequent inhibition of cardiac function,26 and adverse effects were reported in the setting of pressure overload, whereas a protective role was suggested with respect to MI and β‐adrenergic overstimulation.25, 34
Taken together, we propose symptoms of LH as a screening target for subclinical depression after MI with the potential of distinct myocardial signalling. Intervention strategies have been shown to successfully modify negative reactions to situational stressors35 and may be applied accordingly by health care professionals after specialized training. Because psychopharmacological treatment has not been shown to impact patient outcome convincingly7, 8, 9, 10 and is associated with problematic side effects36 as well as drug interactions, a preventive treatment strategy is not warranted. Further mechanistical studies are necessary to unravel treatment options based on molecular pathophysiology.
The interpretation of behavioural testing for individual symptoms of depression in animals understandably suffers from several limitations and can only be an approximation. Feelings of sadness, guilt, or suicidal thoughts are lost by default.37 However, it is noteworthy that patients with depression also cannot display all possible symptoms, due to their antagonistic nature (e.g., weight gain/loss). Sucrose preference testing may yield divergent results depending on the concentration of sucrose and the duration of testing.14, 38 The tail suspension and Porsolt forced swim test are of established predictive validity but naturally devoid of face and construct validity.39 Conversely, the LH paradigm as a model of depression features good face, construct, and predictive validity. However, the induced depressive‐like state only lasts briefly,16 and female C57BL6 mice were shown to be unsusceptible.40 Also, there is a symptomatic overlap between depression and anxiety.37 The behavioural assays performed here to evaluate anxiety‐like behaviour are limited by a competing overlap with novelty seeking as well as overall motility.
In conclusion, we found MI‐induced HF not to trigger depressive‐like behaviour in mice per se but to induce a subtle behavioural shift towards a subclinical phenotype in a subgroup of mice subjected to the LH paradigm. We suggest antidepressant consequences of elevated cardiac nr4a1 expression after MI, which has been implicated with divergent regulation in the heart–brain axis before.14 Translating our findings to the psycho‐pathogenesis of depression after MI in patients, we suggest a multiple‐hits model of accumulating aversive events ultimately leading to depression after MI. Symptoms of LH may present a marker of subclinical depression after MI, the impact of which remains to be investigated.
Conflict of interest
None declared.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (BA 2258/2‐1, SFB 1118), the European Commission (FP7‐Health‐2010, MEDIA‐261409), and the Deutsches Zentrum für Herz‐Kreislauf‐Forschung (DZHK; German Centre for Cardiovascular Research) and by the BMBF (Federal Ministry of Education and Research) to J.B. and by the German Cardiac Society (DGK) to T.S., C.S., and B.B.
Acknowledgements
The authors thank P. Nawroth for the opportunity to conduct automated Cobas (Troponin T) analysis in his laboratory, as well as J. Krebs‐Haupenthal, S. Harrack, U. Oehl, and M. Oestringer for excellent technical assistance.
Bruns B., Schmitz T., Diemert N., Schwale C., Werhahn S. M., Weyrauther F., Gass P., Vogt M. A., Katus H., Herzog W., Backs J., and Schultz J.‐H. (2019) Learned helplessness reveals a population at risk for depressive‐like behaviour after myocardial infarction in mice, ESC Heart Failure, 6, 711–722. 10.1002/ehf2.12440.
Contributor Information
Bastian Bruns, Email: Bastian.bruns@med.uni-heidelberg.de.
Johannes Backs, Email: Johannes.backs@med.uni-heidelberg.de.
References
- 1. Guha K, McDonagh T. Heart failure epidemiology: European perspective. Curr Cardiol Rev 2013. May; 9: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doehner W, Ural D, Haeusler KG, Celutkiene J, Bestetti R, Cavusoglu Y, Pena‐Duque MA, Glavas D, Iacoviello M, Laufs U, Alvear RM, Mbakwem A, Piepoli MF, Rosen SD, Tsivgoulis G, Vitale C, Yilmaz MB, Anker SD, Filippatos G, Seferovic P, Coats AJS, Ruschitzka F. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 2018; 20: 199–215. [DOI] [PubMed] [Google Scholar]
- 3. Gustad LT, Laugsand LE, Janszky I, Dalen H, Bjerkeset O. Symptoms of anxiety and depression and risk of heart failure: the HUNT Study. Eur J Heart Fail 2014; 16: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geiser F, Urbach AS, Harbrecht U, Conrad R, Potzsch B, Amann N, Kiesewetter K, Sieke A, Wolffs K, Skowasch D. Anxiety and depression in patients three months after myocardial infarction: association with markers of coagulation and the relevance of age. J Psychosom Res 2017; 99: 162–168. [DOI] [PubMed] [Google Scholar]
- 5. Ramos S, Prata J, Bettencourt P, Goncalves FR, Coelho R. Depression predicts mortality and hospitalization in heart failure: a six‐years follow‐up study. J Affect Disord 2016; 201: 162–170. [DOI] [PubMed] [Google Scholar]
- 6. Junger J, Schellberg D, Muller‐Tasch T, Raupp G, Zugck C, Haunstetter A, Zipfel S, Herzog W, Haass M. Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail 2005; 7: 261–267. [DOI] [PubMed] [Google Scholar]
- 7. O'Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, Krishnan R, SADHART‐CHF Investigators . Safety and efficacy of sertraline for depression in patients with heart failure:Results of the SADHART‐CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010; 56: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lesperance F, Frasure‐Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297: 367–379. [DOI] [PubMed] [Google Scholar]
- 9. Angermann CE, Gelbrich G, Störk S, Gunold H, Edelmann F, Wachter R, Schunkert H, Graf T, Kindermann I, Haass M, Blankenberg S, Pankuweit S, Prettin C, Gottwik M, Böhm M, Faller H, Deckert J, Ertl G, for the MOOD‐HF Study Investigators and Committee Members . Effect of escitalopram on all‐cause mortality and hospitalization in patients with heart failure and depression: the MOOD‐HF randomized clinical trial. JAMA 2016; 315: 2683–2693. [DOI] [PubMed] [Google Scholar]
- 10. Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289: 3106–3116. [DOI] [PubMed] [Google Scholar]
- 11. Bah TM, Benderdour M, Kaloustian S, Karam R, Rousseau G, Godbout R. Escitalopram reduces circulating pro‐inflammatory cytokines and improves depressive behavior without affecting sleep in a rat model of post‐cardiac infarct depression. Behav Brain Res 2011; 225: 243–251. [DOI] [PubMed] [Google Scholar]
- 12. Shi S, Liang J, Liu T, Yuan X, Ruan B, Sun L, Tang Y, Yang B, Hu D, Huang C. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: evidence from an animal experiment. PLoS One 2014; 9: e101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tao LY, Huang MY, Saroj T, Wang JN, Wu SZ, He F, Huang KY, Xue YJ, Lingwei J, Liao LM, Tang JF, Ji KT. Effects of macrophage migration inhibitory factor on cardiac reperfusion injury in mice with depression induced by constant‐darkness. J Affect Disord 2017; 229: 403–409. [DOI] [PubMed] [Google Scholar]
- 14. Frey A, Popp S, Post A, Langer S, Lehmann M, Hofmann U, Siren AL, Hommers L, Schmitt A, Strekalova T, Ertl G, Lesch KP, Frantz S. Experimental heart failure causes depression‐like behavior together with differential regulation of inflammatory and structural genes in the brain. Front Behav Neurosci 2014; 8: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Czeh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Progress in Prog Neuropsychopharmacol Biol Psychiatry 2016; 64: 293–310. [DOI] [PubMed] [Google Scholar]
- 16. Chourbaji S, Zacher C, Sanchis‐Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive‐like states in mice. Brain Res Brain Res Protoc 2005; 16: 70–78. [DOI] [PubMed] [Google Scholar]
- 17. Vollmayr B, Gass P. Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res 2013; 354: 171–178. [DOI] [PubMed] [Google Scholar]
- 18. Weinreuter M, Kreusser MM, Beckendorf J, Schreiter FC, Leuschner F, Lehmann LH, Hofmann KP, Rostosky JS, Diemert N, Xu C, Volz HC, Jungmann A, Nickel A, Sticht C, Gretz N, Maack C, Schneider MD, Grone HJ, Muller OJ, Katus HA, Backs J. CaM Kinase II mediates maladaptive post‐infarct remodeling and pro‐inflammatory chemoattractant signaling but not acute myocardial ischemia/reperfusion injury. EMBO Mol Med 2014. 6: 1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolk MV, Meyberg D, Deuse T, Tang‐Quan KR, Robbins RC, Reichenspurner H, Schrepfer S. LAD‐ligation: a murine model of myocardial infarction. EMBO Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plaschke K, Feindt J, Djuric Z, Heiland S, Autschbach F, Lewicka S, Martin E, Bardenheuer HJ, Nawroth PP, Bierhaus A. Chronic corticosterone‐induced deterioration in rat behaviour is not paralleled by changes in hippocampal NF‐κB‐activation. Stress 2006; 9: 97–106. [DOI] [PubMed] [Google Scholar]
- 21. Nikendei C, Schafer H, Weisbrod M, Huber J, Geis N, Katus HA, Bekeredjian R, Herzog W, Pleger ST, Schultz JH. The effects of mitral valve repair on memory performance, executive function, and psychological measures in patients with heart failure. Psychosom Med 2016; 78: 432–442. [DOI] [PubMed] [Google Scholar]
- 22. Gathright EC, Goldstein CM, Josephson RA, Hughes JW. Depression increases the risk of mortality in patients with heart failure: a meta‐analysis. J Psychosom Res 2017; 94: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frasure‐Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D; Atrial Fibrillation and Congestive Heart Failure Investigators. Elevated depression symptoms predict long‐term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation 2009; 120: 134–140. [DOI] [PubMed] [Google Scholar]
- 24. Sokoreli I, Pauws SC, Steyerberg EW, de Vries GJ, Riistama JM, Tesanovic A, Kazmi S, Pellicori P, Cleland JG, Clark AL. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA‐HF study. Eur J Heart Fail 2018; 20: 689–696. [DOI] [PubMed] [Google Scholar]
- 25. Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer‐Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly‐6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 2014; 114: 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehmann LH, Jebessa ZH, Kreusser MM, Horsch A, He T, Kronlage M, Dewenter M, Sramek V, Oehl U, Krebs‐Haupenthal J, von der Lieth AH, Schmidt A, Sun Q, Ritterhoff J, Finke D, Volkers M, Jungmann A, Sauer SW, Thiel C, Nickel A, Kohlhaas M, Schafer M, Sticht C, Maack C, Gretz N, Wagner M, El‐Armouche A, Maier LS, Londono JEC, Meder B, Freichel M, Grone HJ, Most P, Muller OJ, Herzig S, Furlong EEM, Katus HA, Backs J. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med 2018; 24: 62–72. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Liu X, Zhang D, Chen J, Liu S, Berk M. The effects of apoptosis vulnerability markers on the myocardium in depression after myocardial infarction. BMC Med 2013; 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wann BP, Bah TM, Kaloustian S, Boucher M, Dufort AM, Le Marec N, Godbout R, Rousseau G. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J Psychopharmacol 2009; 23: 451–459. [DOI] [PubMed] [Google Scholar]
- 29. Voinov B, Richie WD, Bailey RK. Depression and chronic diseases: it is time for a synergistic mental health and primary care approach. Prim Care Companion CNS Disord 2013; 15 PCC.12r01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta D, Kurhe Y, Radhakrishnan M. Antidepressant effects of insulin in streptozotocin induced diabetic mice: modulation of brain serotonin system. Physiol Behav 2014; 129: 73–78. [DOI] [PubMed] [Google Scholar]
- 31. Lamkin DM, Lutgendorf SK, Lubaroff D, Sood AK, Beltz TG, Johnson AK. Cancer induces inflammation and depressive‐like behavior in the mouse: modulation by social housing. Brain Behav Immun 2011; 25: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shinoda Y, Tagashira H, Bhuiyan MS, Hasegawa H, Kanai H, Zhang C, Han F, Fukunaga K. Corticosteroids mediate heart failure‐induced depression through reduced σ1‐receptor expression. PLoS One 2016; 11: e0163992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013; 246: 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Medzikovic L, Schumacher CA, Verkerk AO, van Deel ED, Wolswinkel R, van der Made I, Bleeker N, Cakici D, van den Hoogenhof MM, Meggouh F, Creemers EE, Remme CA, Baartscheer A, de Winter RJ, de Vries CJ, Arkenbout EK, de Waard V. Orphan nuclear receptor Nur77 affects cardiomyocyte calcium homeostasis and adverse cardiac remodelling. Sci Rep 2015; 5: 15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smallheer BA, Vollman M, Dietrich MS. Learned helplessness and depressive symptoms following myocardial infarction. Clin Nurs Res 2018; 27: 597–616. [DOI] [PubMed] [Google Scholar]
- 36. Frey A, Saxon VM, Popp S, Lehmann M, Mathes D, Pachel C, Hofmann U, Ertl G, Lesch KP, Frantz S. Early citalopram treatment increases mortality due to left ventricular rupture in mice after myocardial infarction. J Mol Cell Cardiol. 2016; 98: 28–36 [DOI] [PubMed] [Google Scholar]
- 37. Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010; 13: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmes A. Targeted gene mutation approaches to the study of anxiety‐like behavior in mice. Neurosci Biobehav Rev 2001; 25: 261–273. [DOI] [PubMed] [Google Scholar]
- 39. Abelaira HM, Reus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Braz J Psychiatry 2013; 35: S112–S120. [DOI] [PubMed] [Google Scholar]
- 40. Chourbaji S, Pfeiffer N, Dormann C, Brandwein C, Fradley R, Sheardown M, Gass P. The suitability of 129SvEv mice for studying depressive‐like behaviour: both males and females develop learned helplessness. Behav Brain Res 2010; 211: 105–110. [DOI] [PubMed] [Google Scholar]


