Abstract
Thrombocytopenia is a common complication of chronic liver disease and creates clinical challenges for patients who need invasive procedures. Options available to increase platelet counts were previously limited to risk-laden therapies such as platelet transfusions, splenic artery embolization, and transjugular intrahepatic portosystemic shunts. Thrombopoietin (TPO) agonists can augment platelet production through TPO receptor agonism. Three oral TPO agents are currently available to increase platelet counts, and in 2018, 2 of these agents (avatrombopag and lusutrombopag) were approved by the US Food and Drug Administration for the purpose of increasing platelet counts in patients with chronic liver disease prior to an invasive procedure. This article summarizes the pathophysiology of thrombocytopenia in chronic liver disease, the clinical challenge that thrombocytopenia poses, and the trials that led to the approval of the TPO agonists. Also discussed are the clinical studies that have been the basis for expert opinions and target platelet levels for cirrhotic patients undergoing procedures. A specific platelet count has not demonstrated a decreased bleeding rate in the periprocedural period in randomized, controlled trials, and using TPO agonists is not devoid of risk. However, the newly approved agents have shown no increase in the rate of portal vein thrombosis in this population and have shown promising results for increasing platelet counts.
Keywords: Thrombocytopenia, chronic liver disease, cirrhosis, thrombopoietin agonists
A common complication of portal hypertension from chronic liver disease (CLD) is thrombocytopenia. The prevalence of thrombocytopenia in patients with liver disease ranges from 6% among patients without cirrhosis to 78% in patients with cirrhosis (Figure).1 Inherently, patients with CLD typically have higher degrees of thrombocytopenia.1 Severe thrombocytopenia (platelet count <50 × 109/L) can contribute to the increased risk of procedural bleeding in patients with CLD, creating a challenge for those who need diagnostic and therapeutic procedures. Procedural bleeding in patients with CLD generates both direct and indirect costs due to blood count monitoring, cancellation or postponement of procedures, hospitalizations, and decreased quality of life.2
Figure.
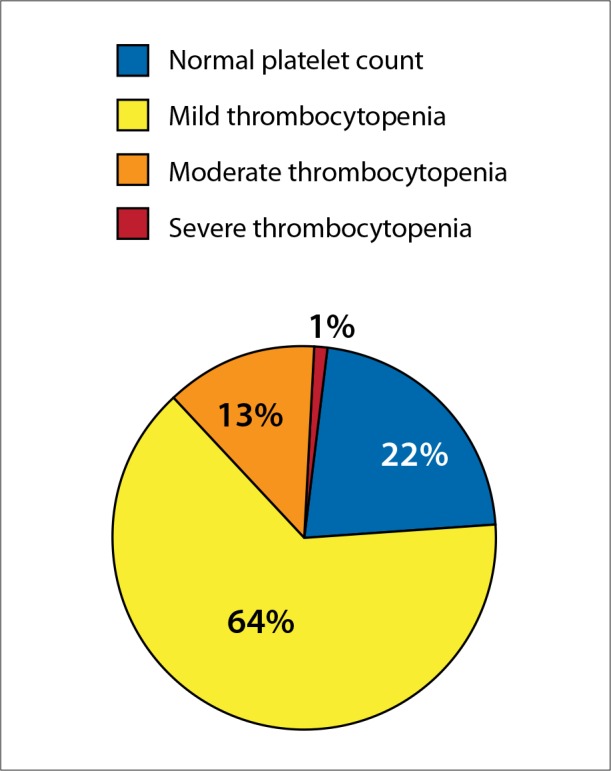
Platelet levels in patients with cirrhosis. Mild thrombocytopenia is defined as a platelet count of 100-150 × 109/L, moderate thrombocytopenia as 50-100 × 109/L, and severe thrombocytopenia as <50 × 109/L.
Pathophysiology of Thrombocytopenia in Chronic Liver Disease
It is important to elucidate the mechanism of thrombocytopenia in patients with CLD. The pathophysiology of thrombocytopenia in these patients is multifactorial. The 2 major contributory mechanisms are splenic sequestration of platelets, which is a sequela of portal hypertension, and decreased hepatic production of thrombopoietin (TPO). TPO acts on the receptor encoded by the Mpl gene and found on the megakaryocytes and all subsequent progenitor cells to platelets. Therefore, TPO, which is secreted at a constant rate from healthy hepatocytes, functions as the dominant hormonal regulator of platelet production. Along with other cytokines, TPO stimulates thrombopoiesis. In patients with CLD, there is a decrease in the amount of circulating TPO, contributing to a quantitative platelet deficiency.1,3 Although patients with cirrhosis are often thrombocytopenic, it has been demonstrated that cirrhotic patients rarely have unprovoked bleeding when compared with patients on anticoagulants.4 Thrombocytosis in end-stage liver disease is thought to be multifactorial, mediated in part by endothelial dysfunction secondary to endotoxin production,5 as well as reduced levels of ADAMTS13 and elevated amounts of von Willebrand factor in circulation.6
The Clinical Challenge of Thrombocytopenia
Giannini and colleagues demonstrated an association between worsening liver disease, worsening thrombocytopenia, and increased procedural bleeding in a single-center cohort of 122 liver disease patients with a mean Model for End-Stage Liver Disease (MELD) score of 22 who were undergoing orthotopic liver transplantation.7 The patients were divided into 2 groups: those with moderate thrombocytopenia (platelet count <150 × 109/L) and those with severe thrombocytopenia (platelet count <75 × 109/L). Of the entire cohort, 50 patients required a procedure prior to transplantation, and 32 (64%) of these patients had severe thrombocytopenia. Procedural bleeding occurred in 10 of the patients with severe thrombocytopenia, and the procedures included endoscopic polypectomy, dental extraction, transcatheter arterial chemoembolization, and radiofrequency thermal ablation of hepatocellular carcinoma. None of the patients with moderate thrombocytopenia who underwent a procedure experienced procedural bleeding.7 This study showed that patients with worsening degrees of thrombocytopenia, portending worsening liver disease, are more likely to require a procedure and are at risk to bleed from a procedure. Although there is no clear consensus on which measures should be taken prior to procedures and what the target platelet level is, clinicians often select prophylactic therapies in an effort to reduce bleeding complications in the periprocedural period. Previously, the list of prophylactic and therapeutic options to avoid or treat procedural bleeding in patients with CLD was limited and also required careful attention due to potential unwelcome side effects. These options included platelet transfusions, splenic artery embolization, splenectomy, and transjugular intrahepatic portosystemic shunts. Alternative therapeutic options to increase platelet counts have recently become an attractive solution to this clinical problem.
Thrombopoietin Agonists
Augmentation of thrombopoiesis through TPO receptor agonism is an alternative therapeutic method to stimulate platelet production in patients with thrombocytopenia. TPO agonists stimulate the same receptor as TPO, but work at a different locus on the receptor. This synergistic effect helps inherent platelet production and maturation of megakaryocytes.
Eltrombopag
Eltrombopag (Promacta, Novartis) is a first-generation TPO receptor agonist and was studied in the CLD population. Although it gained approval from the US Food and Drug Administration (FDA) for chronic immune thrombocytopenia in adults who are refractory to first-line therapy (corticosteroids, intravenous immunoglobulin, and splenectomy), its use is not recommended in patients with CLD due to the increased risk of venous thromboembolism. Afdhal and colleagues randomized 292 patients with CLD (median MELD score, 12) and thrombocytopenia (platelet counts <50 × 109/L) to receive eltrombopag 75 mg daily vs placebo for a 14-day course prior to a procedure to be performed within 5 days after the last dose.8 In this study, referred to as the ELEVATE trial, 72% of patients treated with eltrombopag met the primary endpoint of increased platelet counts and reduced need for platelet transfusions in the setting of a planned procedure, and 59% of patients treated with eltrombopag had an increase in platelet count to more than 80 × 109/L. However, this study was stopped due to 6 patients in the treatment group and 2 patients in the placebo group developing thrombotic events (odds ratio with eltrombopag, 3.04; 95% CI, 0.62-14.82). Post hoc analysis from this trial suggested an association between a platelet count of over 200 × 109/L and the occurrence of portal vein thrombosis (PVT) events; therefore, avoidance of this threshold is advised.8 The notion of stimulating platelet production using TPO agonism was promising; however, further studies were required to avoid the harmful side effect of this therapeutic approach in CLD patients.
Avatrombopag
Avatrombopag (Doptelet, Dova Pharmaceuticals) is a second-generation TPO receptor agonist that was approved by the FDA on May 21, 2018 for the treatment of severe thrombocytopenia in adult patients with CLD who are undergoing invasive procedures. Avatrombopag acts on the TPO receptor, stimulating pathways to promote stem cell differentiation into megakaryocytes. This drug results in a dose-dependent elevation of platelet count, with onset of platelet increase within 3 to 5 days of initiation of therapy. The current FDA recommendation is to begin taking avatrombopag 10 to 13 days prior to a scheduled procedure, with patients undergoing the procedure within 5 to 8 days after the last dose. The recommended dose of avatrombopag is based on the patient’s platelet count prior to the scheduled procedure. There are no contraindications to this drug; however, clinicians are urged to take caution when considering its use in patients with known risk factors for thromboembolism.
Terrault and colleagues performed 2 double-blinded, randomized, controlled studies to evaluate avatrombopag (ADAPT-1 and ADAPT-2).9 These were identical randomized, controlled trials that included CLD patients who were Child-Turcotte-Pugh (CTP) class A through C, had a median MELD score of 10 to 11, and had severe thrombocytopenia (platelet counts <50 × 109/L). All patients in these studies were projected to undergo a diagnostic or therapeutic procedure and were expected to require a platelet transfusion as part of management. Combined, the ADAPT-1 and ADAPT-2 trials randomized 430 patients to receive either avatrombopag (n=274) or placebo (n=156) daily for 5 days. The primary endpoint of these studies was to avoid platelet transfusion prior to the first invasive procedure and eliminate the need for rescue therapy due to bleeding up to 7 days postprocedure. The procedures ranged from low- to high-bleeding risk, and the proportions of patients undergoing low-, moderate-, and high-risk procedures were similar between the avatrombopag and placebo groups. Secondary endpoints included the percentage of patients who obtained a platelet count of 50 × 109/L on the day of the procedure and the change in platelet count from baseline to the procedure day. The studies stratified dosing of avatrombopag based on baseline platelet count. In the 2 trials combined, 160 patients with platelet counts less than 40 × 109/L received avatrombopag 60 mg daily for 5 days, and 117 patients with platelet counts of at least 40 to 50 × 109/L received avatrombopag 40 mg daily for 5 days. A procedure was performed 5 to 8 days after the completion of therapy. Patients were then followed for 30 days. Results showed that the proportion of patients who were able to avoid platelet transfusions or rescue therapy was significantly higher among patients treated with avatrombopag than those treated with placebo (66%-69% vs 23%-35%). In regard to secondary outcomes, in both baseline platelet groups, significantly more patients in the avatrombopag arm reached a platelet count over 50 × 109/L compared with patients in the placebo arm. Among patients with a lower baseline platelet count, those who received avatrombopag had an average platelet increase of 32.0 × 109/L vs 0.8 × 109/L in those who received placebo in the ADAPT-1 trial, and these groups had an average platelet increase of 31.3 × 109/L vs 3.0 × 109/L, respectively, in the ADAPT-2 trial. Among patients with a higher baseline platelet count, those who received avatrombopag had an average platelet increase of 37.1 × 109/L vs 1.0 × 109/L in those who received placebo in the ADAPT-1 trial, and these groups had an average platelet increase of 44.9 × 109/L vs 5.9 × 109/L, respectively, in the ADAPT-2 trial. A total of 3 patients in both studies combined achieved a platelet count of over 200 × 109/L, and all of these patients were asymptomatic. There was 1 reported thrombosis event, which was considered to be nonserious, in the avatrombopag arm. This patient, a 71-year-old man who underwent upper gastrointestinal endoscopy, developed partial portal thrombosis on day 18 of the trial period. His platelet count peaked at 77 × 109/L on day 10 and returned to baseline level at day 37. Overall, avatrombopag was tolerated, and the most common reported adverse events (>5%) included pyrexia, abdominal pain, nausea, and headache. The ADAPT-1 and ADAPT-2 trials provided clinical evidence that avatrombopag can be safely used to increase platelet counts in patients with CLD who are scheduled to undergo an invasive procedure.
Lusutrombopag
Lusutrombopag (Mulpleta, Shionogi) is a second-generation TPO receptor agonist that was approved by the FDA on July 31, 2018 for the treatment of thrombocytopenia in adult patients with CLD who are scheduled to undergo an invasive procedure. This drug works in a similar manner to avatrombopag and induces platelet production by selective agonism of the human TPO receptor.3 The current FDA recommendations are to begin lusutrombopag dosing 8 to 14 days prior to a scheduled procedure, with patients undergoing the procedure 2 to 8 days after the last dose. The recommended dose is 3 mg daily for 7 days given orally. There are no contraindications to this drug. Lusutrombopag demonstrated dose-proportional pharmacokinetics, and there were no clinically significant differences in the pharmacokinetics based on age, race, high-fat meal, mild-to-moderate hepatic impairment (CTP classes A and B), and mild-to-moderate renal impairment (creatinine clearance >30 mL/min).10
Two phase 3 trials have evaluated the use of lusutrombopag in CLD patients. The first, which was named the L-PLUS 1 trial and was conducted by Hidaka and colleagues, occurred in 81 sites across Japan.11 This trial randomized 96 patients to either placebo or the drug for 7 days. The scheduled procedure was then performed 2 to 7 days after the last dose was given. Follow-up continued for 15 days after the procedure. The trial excluded CTP class C patients and patients with a history of PVT. The primary endpoint was the proportion of patients not requiring platelet transfusion prior to the invasive procedure, with the threshold for platelet transfusion being a preoperative platelet count of less than 50 × 109/L. The study concluded that lusutrombopag significantly reduced the need for platelet transfusion prior to an invasive procedure (79% vs 12%; P<.0001). The median increase in platelet count without transfusion in the lusutrombopag arm was 87 × 109/L. In this study, lusutrombopag was well tolerated without any deaths or discontinuations due to adverse outcomes.11 Two thromboembolic events were recorded from the total of 96 patients, 1 event in each arm of the study, and neither was related to the platelet count.
The success of L-PLUS 1 prompted a global phase 3 clinical trial, L-PLUS 2.12 L-PLUS 2 was a double-blinded, placebo-controlled, phase 3 clinical trial performed in 23 countries that evaluated lusutrombopag in patients with CLD who were scheduled to undergo an invasive procedure. L-PLUS 2 randomized patients to receive either drug or placebo for 7 days, and a scheduled invasive procedure was performed 2 to 7 days after the last dose. Follow-up continued for 15 days after the procedure was completed. The primary endpoint was the proportion of patients who did not require platelet transfusion prior to the first invasive procedure. The study proved that lusutrombopag significantly reduced the need for platelet transfusion compared with placebo (65% did not require transfusion vs 29% with placebo; P<.0001). Similar to the findings from L-PLUS 1, lusutrombopag was safe and well tolerated in this study, with a lower incidence of bleeding-related events compared with placebo. In this trial, a total of 3 thromboembolic events were recorded, 1 in the lusutrombopag arm and 2 in the placebo arm, which were unrelated to platelet count. Overall, based on the L-PLUS 1 and L-PLUS 2 clinical trials, lusutrombopag appears to be another viable alternative to platelet transfusion in CLD patients with thrombocytopenia who are undergoing invasive procedures.
Clinical Implications and Management of Thrombocytopenia in Chronic Liver Disease
There is no consensus in regard to what is the optimal platelet threshold that reduces bleeding risk in patients with CLD who are undergoing an invasive procedure. The 2009 American Association for the Study of Liver Diseases Clinical Practice Guidelines for percutaneous liver biopsy recommend a platelet range of 56 to 60 × 109/L.13 This recommendation is based on expert opinion formed from retrospective data and an in vitro analysis of thrombin generation potential in cirrhotic patients compared with healthy controls.14,15 Tripodi and colleagues performed a study comparing thrombin generation in patients with cirrhosis to healthy individuals, and found that a platelet count of 56 × 109/L in cirrhotic patients was the level at which thrombin generation was comparable to the lower limit of the normal reference range in healthy individuals.15 This study also demonstrated that in order to achieve values of thrombin generation equal to healthy subjects, a platelet count of 100 × 109/L was required in cirrhotic patients.
Recent Research
More recent data from post hoc analysis of the HALT-C trial showed an increase in the relative risk of bleeding in patients with platelet counts of no more than 60 × 109/L who were undergoing percutaneous liver biopsy.16 However, more bleeding events (13 of the total 16) occurred in patients with platelet values higher than 60 × 109/L. These data reinforce the difficulty of defining a clear target to reduce bleeding events. Furthermore, the HALT-C cohort consisted solely of patients with hepatitis C virus infection and excluded patients with a CTP class B or higher (equivalent to a CTP score of >7) at enrollment. Therefore, these results may not truly reflect the risks of periprocedural bleeding in patients with CLD. Napolitano and colleagues found that neither platelet count nor international normalized ratio was predictive of bleeding in a prospective case series involving 852 procedures carried out in 363 cirrhotic patients (124 CTP class A, 154 CTP class B, 85 CTP class C).17
No randomized, controlled trials have demonstrated a clinically significant reduction in the risk of periprocedural bleeding by any intervention to increase the platelet count in patients with CLD. In the absence of such data and no clear target, whether to prophylactically intervene and increase platelet counts in patients with CLD who plan to undergo an invasive procedure should be decided on a case-by-case basis. Factors such as the severity of CLD, the presence of additional bleeding risk factors, and the type of invasive procedure (which can vary in the risk of immediate and delayed bleeding) are often weighed in the decision-making process.
Current Practice
It is common practice for clinicians to target a platelet goal of greater than 50 × 109/L in patients with CLD who are undergoing an invasive procedure. This cutoff is derived largely from the in vitro studies performed by Tripodi and colleagues,4,15 as well as from observational studies that have demonstrated a correlation of thrombocytopenia and the risk of periprocedural bleeding.7,16 The definition of high-risk procedures for periprocedural bleeding is also poorly defined in the literature. In the ADAPT-1 and ADAPT-2 trials, low-risk procedures were defined as paracentesis, thoracentesis, and gastrointestinal endoscopy; moderate-risk procedures were defined as liver biopsy, bronchoscopy, ethanol ablation therapy, and chemoembolization; and high-risk procedures were defined as vascular catheterization, transjugular intrahepatic portosystemic shunt, dental procedures, renal biopsy, biliary interventions, nephrostomy tube placement, radiofrequency ablation, and laparoscopic interventions.9 Even among some of the aforementioned moderate- to high-risk procedures, there are a dearth of data supporting a clear platelet threshold that dictates risk in patients with CLD. Sharma and colleagues reported on the risk of bleeding in patients with CLD who were undergoing right and/or left heart catheterization, and found no significant difference in the rate of hematoma formation and the incidence of intracranial or retroperitoneal hemorrhage between controls and patients with CLD.18 However, major bleeding was seen in more patients with end-stage liver disease than in controls (14.8% vs 3.7%; P=.014). Furthermore, this study found no statistically significant relationship between platelet count and the risk of bleeding. However, the mean platelet count in the CLD group was 90 × 109/L, which is a potential explanation for why this trend was not observed. Wallace and colleagues reported that 30 × 109/L was an acceptable platelet count threshold for patients undergoing transjugular liver biopsy, but this study was performed in patients with hematologic malignancy and thrombocytopenia.19 Therefore, its generalizability to patients with CLD is unclear. Studies on dental extractions in patients with CLD are limited. A recent Brazilian study in patients with CLD who were undergoing dental extractions found that intraoperative bleeding was associated with a low platelet count (P=.026), but MELD was not.20 However, there was a higher incidence of postoperative bleeding in patients with normal platelet values, which again underscores the unpredictable nature of periprocedural bleeding in patients with CLD.
Clinicians may opt to prophylactically transfuse platelets in patients with CLD prior to an invasive procedure. However, this strategy exposes patients to risks of febrile nonhemolytic transfusion reactions, cardiac or pulmonary overload from increased intravascular volume, and alloimmunization. In its most severe form, alloimmunization can result in patients becoming refractory to future transfusions, which can have catastrophic downstream effects in the setting of life-threatening hemorrhage.21 Platelets are often scarce in supply, and the economic effects of prophylactic platelet transfusions are not fully known. A recent study estimated that the financial costs associated with a prophylactic platelet transfusion in a single patient with CLD was in the range of $5258 to $13,117 (2017 US dollars).22 There is evidence to suggest that platelet transfusion outside of the setting of acute bleeding is not an effective therapy in patients with CLD. In patients with hypersplenism, up to 90% of transfused platelets are immediately sequestered in the spleen.23 In a study of 26 patients with cirrhosis and thrombocytopenia who underwent endoscopic variceal ligation, Tripodi and colleagues demonstrated that transfusion with 1 standard unit of platelets did not significantly change the results of thrombin generation and thromboelastometry testing, and resulted in a modest increase in platelet counts.24
Prior to the approval of second-generation TPO agonists, platelet transfusion provided the least invasive and lowest risk means to increase platelet counts in patients with CLD. This was largely due to the perceived risk of a thrombotic event that was seen with earlier-generation TPO agonists. Results from the phase 3 clinical trials of avatrombopag (ADAPT-1 and ADAPT-2) and lusutrombopag (L-PLUS 1 and L-PLUS 2) demonstrated that these agents are an efficacious and well-tolerated means to elevate platelet counts in patients with CLD who are undergoing invasive procedures.9,11,25 Both agents are currently FDA-approved for this indication (Table 1).
Table 1.
FDA Dosing Recommendations for Thrombopoietin Receptor Agonists
| Agent | Population | Dosing | Timing |
|---|---|---|---|
| Eltrombopag | Contraindicated in cirrhotic patients | N/A | N/A |
| Avatrombopag | Cirrhotic patients with platelet counts 40-50 × 109/L | 40 mg daily for 5 days | Initiate dosing 10-13 days prior to procedure. |
| Cirrhotic patients with platelet counts <40 × 109/L | 60 mg daily for 5 days | Initiate dosing 10-13 days prior to procedure. | |
| Lusutrombopag | Cirrhotic patients with platelet counts <50 × 109/L | 3 mg daily for 7 days | Initiate dosing 8-14 days prior to procedure. |
FDA, US Food and Drug Administration; N/A, not applicable.
The ADAPT-1 and ADAPT-2 trials did not demonstrate any differences in bleeding between patients who received placebo vs avatrombopag; however, neither trial was sufficiently powered to detect this difference. Published comprehensive details regarding bleeding from the L-PLUS 1 and L-PLUS 2 trials are limited. Reported bleeding-related events were lower in patients who received lusutrombopag compared with placebo in both studies, but it is unclear whether these differences were statistically significant.11,25
A clinically important effect provided by TPO agonists is the prolonged elevation of platelet counts. For example, in the L-PLUS 1 trial, the median number of days with a platelet count of at least 50 × 109/L was 22 in patients who received lusutrombopag compared with 3.3 in the placebo group. This prolonged duration of effect may be particularly appealing in clinical scenarios where there is concern for delayed procedural bleeding, as is the case with postpolypectomy bleeding or endoscopic variceal ligation ulcer bleeding.
Summary
In patients with CLD who are undergoing an invasive procedure, there is no widely accepted platelet count to target. Periprocedural platelet transfusion is a commonly used strategy in patients thought to be at risk for bleeding complications due to their thrombocytopenia. However, this exposes patients to transfusion-related adverse events, as well as the possibility of becoming refractory to platelet transfusion. Avatrombopag and lusutrombopag, second-generation TPO agonists, provide an alternative means of increasing platelet counts in patients with CLD who are undergoing invasive procedures, with the added benefit of prolonged effect. Prior to utilizing a TPO agonist, an evaluation of the patient’s risk for a thrombotic event is strongly recommended (Table 2). Although not required by the package insert of either medication, we advocate for the use of hepatic ultrasound with Doppler to assess patency of the hepatic vessels and evaluate flow in the portal vein prior to TPO agonist use.
Table 2.
Thrombotic Risk With Thrombopoietin Receptor Agonists
| Agent | Thrombotic Risk |
|---|---|
| Eltrombopag | Increased risk compared with placebo |
| Avatrombopag | Same as placebo |
| Lusutrombopag | Same as placebo |
References
- 1.Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37(6):778–793. doi: 10.1111/liv.13317. [DOI] [PubMed] [Google Scholar]
- 2.Brown RS., Jr Review article: a pharmacoeconomic analysis of thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26(suppl 1):41–48. doi: 10.1111/j.1365-2036.2007.03505.x. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi K, Patel S, Meillier A. The use of thrombopoietin receptor agonists for correction of thrombocytopenia prior to elective procedures in chronic liver diseases: review of current evidence. Int J Hepatol. 2016;2016:1802932. doi: 10.1155/2016/1802932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripodi A, Salerno F, Chantarangkul V et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41(3):553–558. doi: 10.1002/hep.20569. [DOI] [PubMed] [Google Scholar]
- 5.Violi F, Ferro D, Basili S et al. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost. 1997;77(1):44–47. [PubMed] [Google Scholar]
- 6.Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98(9):2730–2735. doi: 10.1182/blood.v98.9.2730. [DOI] [PubMed] [Google Scholar]
- 7.Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8(10):899–902. doi: 10.1016/j.cgh.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Afdhal NH, Giannini EG, Tayyab G et al. ELEVATE Study Group. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367(8):716–724. doi: 10.1056/NEJMoa1110709. [DOI] [PubMed] [Google Scholar]
- 9.Terrault N, Chen YC, Izumi N et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155(3):705–718. doi: 10.1053/j.gastro.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Katsube T, Ishibashi T, Kano T, Wajima T. Population pharmacokinetic and pharmacodynamic modeling of lusutrombopag, a newly developed oral thrombopoietin receptor agonist, in healthy subjects. Clin Pharmacokinet. 2016;55(11):1423–1433. doi: 10.1007/s40262-016-0411-6. [DOI] [PubMed] [Google Scholar]
- 11.Hidaka H, Kurosaki M, Tanaka H et al. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin Gastroenterol Hepatol. 2019;17(6):1192–1200. doi: 10.1016/j.cgh.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 12.Peck-Radosavljevic M, Simon K, Iacobellis A et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L-PLUS 2) [published online February 14, 2019] Hepatology. doi: 10.1002/hep.30561. doi:10.1002/hep.30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, McDonald GB, Banaji M. The risk of bleeding after percutaneous liver biopsy: relation to platelet count. J Clin Gastroenterol. 1982;4(5):451–453. doi: 10.1097/00004836-198210000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Tripodi A, Primignani M, Chantarangkul V et al. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44(2):440–445. doi: 10.1002/hep.21266. [DOI] [PubMed] [Google Scholar]
- 16.Seeff LB, Everson GT, Morgan TR et al. HALT-C Trial Group. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8(10):877–883. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Napolitano G, Iacobellis A, Merla A et al. Bleeding after invasive procedures is rare and unpredicted by platelet counts in cirrhotic patients with thrombocytopenia. Eur J Intern Med. 2017;38:79–82. doi: 10.1016/j.ejim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M, Yong C, Majure D et al. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103(5):742–746. doi: 10.1016/j.amjcard.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Wallace MJ, Narvios A, Lichtiger B et al. Transjugular liver biopsy in patients with hematologic malignancy and severe thrombocytopenia. J Vasc Interv Radiol. 2003;14(3):323–327. doi: 10.1097/01.rvi.0000058413.01661.b9. [DOI] [PubMed] [Google Scholar]
- 20.Medina JB, Andrade NS, de Paula Eduardo F et al. Bleeding during and after dental extractions in patients with liver cirrhosis. Int J Oral Maxillofac Surg. 2018;47(12):1543–1549. doi: 10.1016/j.ijom.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Maan R, de Knegt RJ, Veldt BJ. Management of thrombocytopenia in chronic liver disease: focus on pharmacotherapeutic strategies. Drugs. 2015;75(17):1981–1992. doi: 10.1007/s40265-015-0480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnett CL, Mladsi D, Vredenburg M, Aggarwal K. Cost estimate of platelet transfusion in the United States for patients with chronic liver disease and associated thrombocytopenia undergoing elective procedures. J Med Econ. 2018;21(8):827–834. doi: 10.1080/13696998.2018.1490301. [DOI] [PubMed] [Google Scholar]
- 23.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45(5):645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripodi A, Primignani M, Chantarangkul V et al. Global hemostasis tests in patients with cirrhosis before and after prophylactic platelet transfusion. Liver Int. 2013;33(3):362–367. doi: 10.1111/liv.12038. [DOI] [PubMed] [Google Scholar]
- 25.Afdhal N, Duggal A, Ochiai T et al. Platelet response to lusutrombopag, a thrombopoietin receptor agonist, in patients with chronic liver disease and thrombocytopenia undergoing non-emergency invasive procedures: results from a phase 3 randomized, double-blind, placebo-controlled study. Blood. 2017;130(suppl 1):291. [Google Scholar]


