Abstract
Soft tissue sarcomas (STS) are rare tumors; they do not even equate to 1% of all malignant tumor cases. One-fifth of all STS occur in the upper extremities, where epithelioid sarcoma, synovial sarcoma, clear cell sarcoma and malignant fibrohistiocytoma are the most frequent subtypes. Surgical resection is the cornerstone of treatment. However, accomplishment of optimal oncological and functional results of STS of the upper extremities may represent a challenge for hand surgeons, due to the complex anatomy. In several cases, preoperative therapies are needed to facilitate tumor resection and improve the oncological outcome. Oligometastatic disease may also be a challenging scenario as curative strategies can be applied. Radiotherapy and chemotherapy are commonly used for this purpose albeit with conflicting evidence. Novel drug combinations have also been approved in the metastatic setting, further improving the quality of life and survival of eligible patients. Thus, prior to any approach, every case should be individually discussed in sarcoma centers with specialized multidisciplinary tumor boards. The aim of the present review was to gather the multidisciplinary experiences of the available therapeutic strategies for STS of the upper extremities.
Keywords: soft tissue sarcomas of the extremities, hand, limb sparing surgery, adjuvant therapy, chemotherapy, radiotherapy, metastases
1. Introduction
Soft tissue sarcomas (STS) constitute a heterogenic group of tumors that accounts for only 1% of the overall human burden of malignant tumors, with an annual incidence of approximately 4.5/100,000 in Europe (1). Around 70 to 80% of patients are diagnosed in a local or locally advanced stage of the disease. The median age at diagnosis is 58 years, and the STS-related death around 65 years (2).
Extremities are the most frequent location for STS, accounting for 21.7% of all STS. However, a lower incidence (25–30% of STS of the extremities) and an earlier median age of diagnosis (38 years, ranging from 4 to 77 years) have been reported for the upper extremities compared to lower extremities' STS (1). Around 50% of STS of upper extremities arise in the shoulder-upper arm region, 30 to 40% in the elbow-forearm and only a 10 to 20% in the wrist-hand (3,4). However, when the upper extremity is subdivided in proximal and distal, a distribution of 50% in each site has been observed (5) (Table I).
Table I.
Description of studies reporting on frequency and location of STS of the upper extremity.
| Authors, year | Total | Shoulder-Arm, n (%) | Elbow-Forearm, n (%) | Wrist-Hand, n (%) | (Refs.) |
|---|---|---|---|---|---|
| Gustafson and Arner, 1999 | 108 | 50 (46.3) | 48 (44.4) | 10 (9.2) | (3) |
| Gerrand et al, 2003 | 139 | 74 (53.2) | 41 (29.5) | 24 (17.3) | (4) |
| Müller et al, 2016 | 195 | 98 (50.2) | 97 (49.8) | (5) | |
| Total | 442 | Proximal: 222 (50.2) | Distal: 220 (49.8) | – | |
STS, soft tissue sarcomas.
2. Histological examination
Over 50 different histological and molecular subtypes have been described, occurring ubiquitously throughout the human body (6). As every subtype of sarcoma has a particular biological behavior and response profile to systemic therapy, histologic diagnosis is a crucial criterion when selecting the appropriate therapy. Distribution of these subtypes varies between registries, due to evolution of classification of STS as a result of histological and molecular biology advances (6). Potentially, all histologic subtypes can arise on the upper extremities, but a higher incidence of malignant histiocytofibrosarcoma/undifferentiated pleomorphic sarcoma (UPS) and synovial sarcoma (SS) has been reported, reaching 50–65% (7). Of note is that epithelioid sarcoma (ES) arises almost exclusively on the extremities, while clear cell sarcoma (CCS) is considered a specific subtype of the hand and the wrist (2). Histological, immunohistochemical and cytogenetical characteristics of the specific subtypes of STS of the upper extremities are described in Table II.
Table II.
Histological, immunohistochemical and cytogenetical characteristics of the more frequent subtypes of STS of the upper extremities.
| Sarcoma type | Histology | IHC | Cytogenetics |
|---|---|---|---|
| UPS | Cytological and nuclear pleomorphism | Positivity for antigens suggesting diverse lines of differentiation in the same tumor. | Great number of genetic alterations. Phenotypic spectrum of a single molecular entity with myxoid fibrosarcoma. |
| SS | Biphasic: Spindle cell component with an epithelial component. Monophasic: Entirely compounded by the spindle component. | CK, EMA, S100, TLE1 positive. | Translocation t(X;18)(p11;q11) (90% of cases); fusion gene SSX-SYT. |
| ES | Epithelial and spindle cells that form nodules. | Vimentin, CK, EMA positive. SMARCB/INI1 negative. | No conclusions about the genetic aberrations can be drawn due to the low incidence of this tumor. |
| CCS | Spindle or polygonal cells with abundant cytoplasm disposed in nests with fibrous tracts between them. | Vimentin, HMB-45, S100, Melan-A positivity | Translocation t(12;22)(q13;q12); fusion gene EWS-ATF1. |
All data were obtained from reference (6). STS, soft tissue sarcomas; UPS, undifferentiated pleomorphic sarcoma; SS, synovial sarcoma; ES, epithelioid sarcoma; CCS, clear-cell sarcoma; IHC, immunohistochemistry.
Accurate pathologic characterization of STS requires adequate and representative tumoral tissue, such as that harvested by core needle biopsy (CNB), which attains a specificity of 70 to 98% (8). The most accepted grading system for STS is the FNCLCC (Fédération Nationale des Centres de Lutte Contre le Cancer) system, based on three scores: Differentiation, mitotic count and necrosis. STS of the upper extremities are categorized as high grade in 45–70% of cases, due to the high score attributed to each of the four most frequent but aggressive histologic subtypes (3,9).
3. Diagnostic approach
Most STS of the hand and upper extremities present as a painless, slow growing and movable mass. In rare cases, the mass may cause nerve compression and present clinically as a nerve compression neuropathy. Thus, malignant lesions are ill-conceived as benign tumors and treated inadequately (10). Although the most common soft tissue histology of the upper extremities is lipoma, superficial soft tissue tumors larger than 5 cm or deep-seated tumors are associated with a higher risk of malignancy. Within the 3–5 cm size category, akin to a golf ball (4.3 cm), the risk of malignancy is influenced by increasing age (11).
Acral myxoinflammatory fibroblastic sarcoma, ES and CCS are frequently located in the hand and often present as painless slow growing nodules, commonly confused with wrist ganglions and erroneously treated accordingly. As these tumors are solid and tend to extend along tendon sheaths, narrow resections may result in high recurrence rates. Contrary to other subtypes, CCS and ES have a high rate of regional node involvement (12,13).
Magnetic resonance imaging (MRI) is the method of choice for the radiological evaluation of suspicious lumps, informing on the anatomical relations with the surrounding tissues for optimal surgical planning (14). Despite established imaging criteria, precise diagnosis can be made on the basis of MRI in only 24% of cases (15). Gadolinium contrast administration provides important information on tumor heterogeneity, guiding biopsy to the most vascular, non-necrotic part of the lesion (16).
A percutaneous core 14G-needle biopsy is frequently performed under local anesthesia. Through a skin stub 3–4 tissue cylinders are harvested. This procedure allows to harvest adequate tissue volume to make the diagnosis in over 90% of cases, with a sensitivity of 95% for malignancy and 88% for grade (17). Nevertheless, major diagnostic errors associated with the use of CNB can be drawn due to tumor heterogeneity and low cellularity in cases of lipomatous, hemorrhagic or myxoid tumors. More specifically, in dedifferentiated sarcomas, low-grade and high-grade components coexist in the same mass and a biopsy taken from the low-grade part may therefore result in underestimation of the true tumor grade. In order to increase the harvesting of representative tissue, the careful consideration of the MRI features and the accomplishment of CNB under CT or U/S guidance are recommended.
When CNB is repeatedly non-diagnostic, an open biopsy should be performed, as it has a diagnostic accuracy of 94–100%. Open biopsies should be carefully planned and performed. The incision line should be part of the final surgical approach. We avoid transverse incisions as they usually create a soft tissue defect difficult to reconstruct after final resection of the tumor with the biopsy tract. The surgeon should be aware of the complex nerve and vessel anatomy of the upper extremity. The biopsy tract must not violate more than one anatomic compartment and avoid exposure of the neurovascular bundles to the tumor cells. The pseudocapsule of the tumor should be closed with sutures after tissue harvesting. Adequate hemostasis should be performed in order to avoid hematoma formation and when a draining tube is placed it should be in line and close to the incision.
Despite its higher diagnostic value, open biopsy is kept as the last resource as it is expensive, carry a complication rate of up to 16% and may cause contamination of the incisional path. At the final surgery for tumor resection, the surgical path of the biopsy (including 1–3 cm of the skin around the incision and subcutaneous tissues) should be excised en block with the final tumor specimen (18).
In case of small superficial lesions, well planned excisional biopsies with negative margins can be performed (19). An absolute prerequisite to decide for an excisional biopsy is the ability to resect the mass with negative histology margins. The surgeon performing an excisional biopsy should have measured in detail on pre-op MRI the size of the lesion and the relation to surrounding tissue. An exception to this concept, is the benign giant cell tumor of tendon sheath located in the fingers, where frequently a marginal resection is performed that may result in a higher local recurrence rate (20). In contrast to well-planned excisional biopsy, the ‘unplanned excision’ is defined as the gross removal of tumor without pre-operative staging or consideration for the need for removal of normal tissue around the tumor (21). Unplanned excisional biopsies of the upper extremity, frequently leave microscopic residual disease requiring a more aggressive and debilitating subsequent treatment in up to 45% of cases (22).
Once the diagnosis of malignancy is suspected or established, staging is preferably performed by computed tomography (CT) of the thorax and abdomen whereas positron-emission tomography (PET) is reserved for selected cases (23).
4. General considerations on therapeutic approach
Compared to tumors of the lower limb, upper extremity tumors tend to be smaller, more often superficial (3) and more likely to undergo unplanned excision (24). Oncological surgery represents a major challenge in most cases. The complex and intimate surgical anatomy of tendons, vessels and nerves jeopardizes both the success of appropriate surgical margins and the postoperative loss of function (25). An unplanned excision of upper-limb tumors tends to have a higher rate of positive surgical margins, along with a significant higher rate of local recurrence when compared to lower-limb tumors (3).
The addition of adjuvant chemotherapy (26) and radiotherapy (27) has been reported to improve the outcomes of surgery in unselected patients with STS. As a result, limb-sparing surgery is performed in around 90% of patients with local recurrence rates similar to those observed following amputation (28). Centralized, multidisciplinary diagnosis and treatment in dedicated, high-volume centers is directly related to significantly better survival rates and quality of care for patients, underlying the importance of sarcoma centers of excellence (29).
5. Treatment of localized disease
Surgery
Until late 1970s, amputation was regularly performed for localized STS of the extremities, on account of higher rates of local recurrence (30). Enneking et al (31), described four types of resection margins: Intralesional, marginal within the reactive zone, wide with a cuff of normal tissue and radical resection involving excision of the entire anatomical compartment. Through decades, we moved from the surgical principle of resection of the involved anatomic compartment, to tumor free resection margins as a minimum of acceptable resection. Although a wide soft tissue envelop in all directions around the tumor is desirable, the feasible goal is resection to negative margins (1 mm from the inked resection margin) (32). Still, for STS of the hand, amputation of a digit may be necessary to obtain clear margins.
Currently more than 90% of STS can be treated with local resection and limb salvage. However, a primary amputation should be considered when the tumor cannot be excised to clear margins, based on the pre-op imaging. Extensive soft tissue infiltration and/or involvement of a major neurovascular bundle frequently result in amputation. A primary amputation should be considered when tumor resection results in significant loss of soft tissue with severe function impairment, that cannot be reconstructed with available surgical techniques, or the expected complication rate will be high.
Suboptimal biopsies and positive resection margins are associated with local and distant disease recurrence in patients with hand STS (22). Pradhan et al (33), reported on 63 patients with hand STS. Six patients underwent below elbow amputation and 12 patients had partial amputation. All the amputated patients had clear margins, while 42% out of patients with local tumor excision had involved margins (33). Single ray amputations (excluding thumb) for hand tumors have a low local recurrence rate and high functional scores (34). However, ray transposition should not be performed with ray amputation for tumor excision. In order to achieve negative margins, wider resection may be needed. Double ray amputation results in worse functional outcome than single ray. Good key, tip and tripod pinch can nonetheless be maintained when the deep motor branch of the ulnar nerve is preserved, and this hand can still assist in bimanual hand activities (35).
Preoperative radiation therapy (RT) is useful in cases where the tumor mass is in contact to nerves and vessels, as it may facilitate negative margin resection by inciting a thicker reactive fibrous tumor pseudocapsule, which can be dissected from the neurovascular bundle (36). Clarkson et al (37), concluded that meticulous sharp epineural dissection of the ischial nerve combined with RT is a safe technique and nerve preservation can be attempted when the tumor does not encase the nerve trunk. Although there are no randomized studies available, RT and epineural dissection is the common practice for STS of the upper extremity abutting on major nerves.
When the tumor mass surrounds important vessels, limb-sparing surgery can be performed as an en bloc resection of the sarcoma and vessels with vascular reconstruction. For large diameter vessel reconstruction, the great saphenous vein is usually harvested, reversed and anastomosed proximally and distally to restore anatomic continuity and circulation (38).
Contact of the mass with the bone is a common finding in MRI of large, deep STS and invasion of the bone cortex can be found in cases of SS and UPS. Cortical and medullary signal intensity changes and cortical destruction observed on T1 and T2-weighted MR images are highly sensitive and specific signs of osseous invasion by STS (39). A study from Mount Sinai reported that true bone invasion occurs in a 5.5% and it is associated with increased metastatic disease at presentation and decreased overall survival (OS) (40). Preoperative RT enables resection of the mass with periosteum serving as the deep margin, without expecting increased recurrence rate (41). For STS invading the bone, or when negative margins cannot be achieved using periosteum as a deep margin, en bloc resection of the soft tissue mass with the affected bone should be performed. For segmental bone defects, reconstruction can be done with either avascular bone autograft, allograft, a vascularized fibula graft or a hybrid reconstruction of an allograft combined with a free vascularized fibula graft.
Flap reconstruction is an essential part of STS surgical treatment (42). Tensionless primary wound closure is important to avoid wound healing complications, especially if preoperative RT has been administered. For small size soft tissue defects, wound closure can be achieved either by simple sutures or muscle approximation and split thickness skin grafting. Flap coverage is essential in the case of exposed vessels, nerves or bone. Flap usage is also important in the prevention or management of wound healing complications (43). Frequently used flaps are the lateral arm flap, the radial forearm, anterior-lateral thigh and latissimus dorsi flap (42). Vasileios et al (16) reported on 57 patients with soft tissue malignant fibrous histiocytoma. A rotational or free flap was eventually needed in 28 patients. A major wound complication occurred in 17% of patients. All complications were related to preoperative RT and 90% involved the lower limbs. Wound breakdown was associated with infection in 50% of cases (16).
Prognostic factors
Metastatic relapse after complete surgery occurs in around 40% of patients, leading to death from the disease within the first 8 years after initial diagnosis (44). Several prognostic factors have been identified to assess the probability of recurrence after surgery. High histological grade, size >5 cm, deep location and positive surgical margin status have been characterized as the most important poor prognostic factors (45). Of them, histological grade has been pointed out as the factor with the heaviest prognostic impact for systemic control after surgery (46), while surgical margin status has been described as the most important factor for local control (47). In order to reduce the high probability of relapse, complementary radiation therapy and chemotherapy may be applied.
Radiation therapy
Radiation therapy (RT) is a crucial adjunct to surgery for STS of the extremities. The most important outcome by the use of RT is the local control of the disease, but this is not associated with a significant reduction in distant metastasis or improvement in disease-specific survival (48). There are several RT modalities applied such as external beam radiation therapy (EBRT), intensity-modulated radiation therapy (IMRT), intraoperative radiation therapy (IORT) and brachytherapy.
External beam radiation therapy
The administration of preoperative or adjuvant EBRT in order to avoid amputation is supported by evidence reported from several clinical trials. Selected randomized prospective clinical trials are shown in Table III. The addition of EBRT after LSS attains similar results as amputation and significantly reduces the local-recurrence rate of LSS alone (27). The benefit of adjuvant EBRT seems higher for STS with poor prognostic factors, and data suggest that it might be omitted in patients with completely resected low-risk STS (49,50). Significant differences in toxicity have been reported with the use of postoperative EBRT compared to LSS alone with respect to edema, limb strength and range of motion. Preoperative EBRT, is significantly related to greater acute toxicity and major wound complications than postoperative EBRT, without differences in local-relapse rates and long-term OS (51).
Table III.
Safety and efficacy of selected randomized clinical trials of external-beam radiation therapy in STS of the upper extremities.
| Authors, year | Methods | N | Efficacy outcomes | Safety outcomes | (Refs.) |
|---|---|---|---|---|---|
| Rosenberg et al, 1978 | Amputation vs. LSS/EBRT | 41 | 5y DFS: 78% vs. 71% (P=0.75); 5y OS: 88% vs. 83% | No improvement in quality of life. | (27) |
| Yang et al, 1998 | LSS followed by EBRT vs. no adjuvant treatment | 91 | HGSTS: 10y LRR, 0% vs. 19% (p=0.003); 10y OS: 75% vs. 74%. | Persistent reduction in joint motion. Transient increase in edema and limb weakness. | (49) |
| LGSTS: 10y LRR, 3.8% vs. 33.3% (p=0.016); 10y OS, 3.8% vs 8.3% | |||||
| O'Sullivan et al, 2002 | Preoperative EBRT vs. postoperative EBRT | 190 | 5y LRR 93% vs. 92% (P=0.79); 5y DFS 58% vs. 59% (P=0.83); 5y OS 73% vs. 67% (P=0.48) | Major wound complications: 35% vs. 17% (P=0.01). | (100) |
STS, soft tissue sarcomas; LSS, limb-sparing surgery; EBRT, external-beam radiation therapy; 5y DFS, 5-year disease-free survival; 5y OS, 5-year overall survival; HGSTS, high-grade soft-tissue sarcomas; LGSTS, low-grade soft-tissue sarcomas; 10y LRR, 10-year local recurrence rate; 10y OS, 10-year overall survival; 5y LRR, 5-year local-recurrence rate.
As complex trade-off issues are involved in the sequencing of LSS and RT for patients with localized STS of the extremities, it seems important to define subsets of patients who might be adequately treated by surgery alone and the optimal sequence of surgery and EBRT for patients who require both types of local therapy (52). An attempt has been made to develop a nomogram to quantify the 3- and 5-year risk of local recurrence after LSS without postoperative EBRT that includes age, size, margin status, grade of tumor and histology (53).
Intensity-modulated radiation therapy
The main advantage of IMRT is its ability to deliver high dose RT to the tumor minimizing the dose of RT to the surrounding normal structures. Such a tight margin might compromise tumor coverage and result in a higher rate of local recurrences. A retrospective analysis from the Memorial Sloan Kettering Cancer including 41 patients with poor prognostic factors (34 patients with high grade STS, 21 patients with infiltrated/close <1 mm surgical margins), treated with preoperative (7 patients) or postoperative IMRT (34 patients), reported encouraging data on acute and late toxicity. The 5-year local control rate was 94%, which compares favorably with that of historical controls ranging from 82% in negative margins to 51% in positive margins (54).
A retrospective comparative study including 319 patients with STS of the extremities treated with postoperative EBRT (154 patients) or IMRT (165 patients) with similar dosing schedules indicated that IMRT was associated with significantly reduced local recurrence compared with conventional EBRT (55).
A following prospective phase II study included 80 patients with localized STS of extremities (51 patients) and trunk wall (29 patients). After treatment with function-conserving surgery and postoperative IMRT, an excellent local control was assessed, with low IMRT-associated toxicity such as edema and joint stiffness (56). As a result, IMRT is a promising RT approach in STS of the extremity as it provides excellent local control in a group of patients with high-risk features with a beneficial effect in sparing the surrounding normal tissue.
Intraoperative radiation therapy
Intraoperative RT consists of a single large dose of RT, administered during LSS after tumor removal and prior to wound suturing. As a result, the tumor bed can be irradiated directly sparing the surrounding normal tissue. IORT is usually combined with postoperative RT, but special hospital infrastructures are required. IORT used as a boost to EBRT seems to provide excellent local control with only mild acute side effects, as indicated by a retrospective study of 17 patients with STS of upper or lower extremities (57).
A more recent retrospective analysis of 61 patients with upper-extremity STS treated with surgery, IORT (12.50 Gy) and EBRT (45–50 Gy), associated this strategy with excellent local control, limb preservation and survival, even in patients with positive margins. Only 4 patients developed RT-associated toxicity (58).
Brachytherapy
Brachytherapy is the direct application of radioactive sources into the tumor bed through catheters, which allows a high dose of RT to the tumor in a more conformal way compared to EBRT. This is translated in shorter treatment periods, fewer side effects and a faster recovery. It permits evaluation at the time of surgery and complications can be avoided by sparing the surrounding tissues (59).
A prospective trial randomized 164 patients to receive either adjuvant brachytherapy or no further RT after complete resection of STS of the extremity (60). The RT was administered by iridium-192 implants, which delivered 42 to 45 Gy over 4 to 6 days. The results indicated that in patients with high-grade histologies brachytherapy provides convenient means to complete RT within a short period with no long-term functional sequelae and with a local control benefit comparable to that obtained with more protracted courses of EBRT. These data suggest that brachytherapy is an effective alternative to EBRT.
Adjuvant and preoperative chemotherapy
Chemotherapy for resectable STS of the extremities has been evaluated in both the adjuvant and the preoperative settings.
Adjuvant chemotherapy
The data of nearly twenty clinical trials, carried out from 1980's to 2008, have been gathered in two different meta-analyses (61,62). Tierney et al (61) showed that adjuvant chemotherapy for unselected patients results in an absolute benefit at 10 years of 4%. When subgroup analysis was carried out, sarcoma of the extremities had an absolute benefit at 10 years of 7% (p=029) (61). In a more recent meta-analysis, Pervaiz et al (62) evaluated data from 1,953 patients receiving postoperative chemotherapy. According to this meta-analysis, administration of adjuvant anthracyclines and ifosfamide leads to a significant reduction in the risk of recurrence and death of 10 and 11%, respectively (62).
Conversely, the largest, phase III, placebo controlled, clinical trial for adjuvant chemotherapy in STS, which randomized 351 patients to receive or not 5 cycles of adriamycin and ifosfamide after surgery, found no significant differences, either in relapse free survival or in OS. However, a clear advantage of chemotherapy can be inferred from the forest plot for extremities, grade 3 and greater size (63).
Preoperative chemotherapy
The role of preoperative chemotherapy was first evaluated retrospectively, showing a positive effect mainly for patients with deep, high-grade tumors over 10 cm (64). Prospectively, a large phase III clinical trial randomized 328 high-risk patients to receive neoadjuvant epirubicin 120 mg/m2 and ifosfamide 9 g/m2 for 3 or 5 cycles (65). It was confirmed that 3 cycles do not worsen survival rates (5-year OS of 68% with 3 cycles versus 70% with 5 cycles), and they are comparable to those seen with the same combination in the adjuvant setting (66). The addition of RT was permitted and subgroup analysis of patients with affected surgical margins showed a local relapse rate of 17% if RT was administered after surgery versus 0% when it was performed before (67).
The unselected populations of the previously mentioned clinical trials constitutes a major hindrance when deciding the best therapeutic option for sarcoma patients. Evidence indicates that chemotherapy is more useful for sarcomas of the extremities and the trunk wall, but no differences between upper and lower limbs have been reported. In the absence of clinical trials with selected populations, chemotherapy for STS of the upper limb should be administered for chemosensitive histologic subtypes when poor prognostic factors are present.
Despite the preoperative treatments described hitherto, some cases are not amenable but with amputation of the extremity. These cases led to the exploration of other methods in an attempt to improve the LSS. The isolated limb perfusion (ILP) consists in the administration of chemotherapy after separating the circulation of a limb from that of the rest of the organism. The introduction of TNF-α in combination with melphalan after some frustrating results of ILP with doxorubicin (68) allowed the LSS in 76% of patients who, otherwise, would have required amputation (69).
Follow-up and recurrence
As for STS of other locations, follow-up of STS of the upper extremities has the objective of controlling the sequelae from the administered treatments as well as the detection of local or metastatic relapse. Thus, follow-up is also an important part of the multidisciplinary approach, since early and late complications from surgery, radiotherapy and chemotherapy have to be diagnosed and treated promptly.
Surveillance for uncovering local and metastatic relapse is a controversial subject. Local recurrence of the upper extremity is easily detected by physical examination and even by self-examination, which calls into question the routine use of image tests of the limb (70). Surveillance periodicity varies between clinical guidelines and it depends on several factors, such as histologic grade and subtype or even the experience of every sarcoma center. Despite the tendency to use high-definition image tests, it seems that chest CT does not add a benefit to the use of simple X-ray in the detection of resectable pulmonary metastases (71). However, no prospective trials have determined to date the best surveillance strategy for the detection of both local and metastatic relapse of STS.
Local relapse of a previously treated STS of the upper extremities may not be amenable to re-excision, but the option of radiation and even re-radiation could be possible in selected cases (72).
Similarly, the therapeutic strategy for metastatic disease depends on the number and the site of metastases, potentially managed with local treatments, and on the histologic subtype, potentially sensitive to systemic therapy. The lung is the most common site of metastases, as up to 80% of metastatic STS present with lung metastases (73). Although prospective and randomized studies are lacking, pulmonary metastasectomy with complete resection of all disease burden may be considered for selected patients. According to different reported series, pulmonary metastasectomy attains a 5-year survival rate of 15 to 50.9% (74). The complete resection of all metastases is the most important prognostic factor, as it duplicates the survival compared to that of incomplete resection (75). The number of metastases has also been indicated as an important prognostic factor, though the maximum number of metastases contraindicating the surgery has not been established (74).
6. Chemotherapy and targeted therapy for metastatic STS of the upper extremities
After multidisciplinary curative treatment, the risk of metastatic relapse within the next 2 years after surgery is as high as 46% (29). Thus, improvement in the treatment of metastatic disease is imperative.
If not amenable to salvage surgical procedures, treatment of metastatic sarcoma is still based primarily on chemotherapy. Despite the intrinsic heterogeneity, clinical trials have often been designed for all histological subtypes taken together. Since the decade of the 80 s, anthracyclines have been the drug class of choice for the first line treatment, attaining a limited response rate of under 25% at conventional dose of 60–75 mg/m2 (76). In order to optimize the effectiveness of treatment, combinations of doxorubicin and ifosfamide have been investigated at different doses. Effectiveness of the combination has been reported superior at conventional doses (doxorubicin 60–75 mg/m2 and ifosfamide up to 9 g/m2) at the expense of a greater toxicity, without significant improvement in OS (77,78). High doses of doxorubicin and ifosfamide have also been tested, resulting in a better clinical benefit and progression-free survival (PFS) with the counterpoint of a mainly hematological greater toxicity (79).
Combination of doxorubicin and olaratumab, showed a significant improvement in OS that could not be confirmed in the recently reported, phase III clinical trial ANNOUNCE (NCT02451943), which did not meet its primary endpoint of OS (80).
Following failure on preoperative, adjuvant or first-line chemotherapy with anthracyclines, therapeutic options for STS include other cytotoxic agents, tyrosine-kinase inhibitors (TKI) and immunotherapy (81). Monotherapy with trabectedin at 1, 5 mg/m2 in a continuous 24-hour infusion every 3 weeks is an approved second line option after anthracyclines, achieving a median PFS of 4.2 months (82). Combinations of the antimetabolite gemcitabine, with docetaxel (83) or dacarbazine (84) achieve a median PFS of 6.2 and 4.2 months, respectively. The TKI pazopanib, targeting the vascular endothelial growth factors (VEGF) 1–3, the PDGFR A and B and mast/stem cell growth factor receptor KIT, was tested in a randomized phase III clinical trial and a median PFS of 4.6 months was found (85).
As several reports have indicated differential responses to the available drugs, the choice of specific therapeutics may vary according to histologic subtypes (Table IV) and toxicity (Table V).
Table IV.
First and second line options for the predominant histological subtypes of STS of the upper extremities.
| Sarcoma type | First line | Second and further lines | Drugs under investigation |
|---|---|---|---|
| UPS | Doxorubicin ± Ifosfamidea | Gemcitabine-Docetaxelb; Ifosfamidec; Trabectedind; Pazopanibe | Pembrolizumabf |
| SS | Doxorubicin ± Ifosfamidea | Ifosfamidec; Trabectedind; Pazopanibe | Tazemetostat |
| ES | Doxorubicin ± Ifosfamidea | Gemcitabine-Docetaxelb; Pazopanibe; Trabectedind | Tazemetostat |
| CCS | – | – | Caffeine-potentiated doxorubicin; Sorafenib; Sunitinib; Tinvatinib |
Doxorubicin 60–75 mg/m2 ± ifosfamide up to 9 g/m2 days 1–3 every 21 days until disease progression or unacceptable toxicity (79).
Gemcitabine 900 mg/m2 on days 1 and 8 + Docetaxel 100 mg/m2 from day 8 every 21 days until disease progression or unacceptable toxicity (83).
Ifosfamide 14 g/m2, continuous infusion for 6 days every 21 days with MESNA until disease progression or unacceptable toxicity (89).
Pazopanib 800 mg/day until disease progression or unacceptable toxicity (82).
Trabectedin 1.5 mg/m2 in 24-h continuous infusion every 21 days until disease progression or unacceptable toxicity (81). STS, soft tissue sarcomas; UPS, undifferentiated pleomorphic sarcoma; SS, synovial sarcoma; ES, epithelioid sarcoma; CCS, clear cell sarcoma.
Table V.
Selection of the toxicities of principal available drugs for soft tissue sarcomas.
| Frequency of toxicities | |||
|---|---|---|---|
| Drug | Very common and common | Uncommon | Rare and very rare |
| Doxorubicin | Myelosuppression, Cardiotoxicity | Dehydration | Tissue necrosis |
| Ifosfamide | Myelosuppression, Hepatotoxicity, Hemorrhagic cystitis, Acute renal failure | Peripheral neuropathy, Stomatitis | CNS toxicity, Dermatitis |
| Gemcitabine | Myelosuppression, Elevation of liver transaminases, Allergic skin rash, Influenza-like symptoms | Interstitial pneumonitis | Anaphylactoid reaction, PRES, Capillary leak syndrome |
| Docetaxel | Myelosuppression, Hypersensitivity, Peripheral neuropathy, Fluid retention | Arthralgia, Elevation of liver transaminases | Cardiotoxicity |
| Trabectedin | Myelosuppression, Elevation of liver transaminases, PPEDS | Capillary leak syndrome, Pulmonary edema | Hepatic failure |
| Pazopanib | Hypothyroidism, Hypertension, Hair color change, Elevation of liver transaminases, Diarrhea | Hypomagnesaemia, Retinal detachment, Cardiotoxicity, Intestine perforation | Thrombotic microangiopathy, Posterior reversible encephalopathy, Pneumonitis |
The following was utilized for the classification of frequency: Very common ≥1/10; common ≥1/100 to <1/10; uncommon ≥1/1,000 to <1/100; rare ≥1/10,000 to <1/1,000; and very rare <1/10,000). CNS, Central nervous system; PRES, Posterior reversible; encephalopathy syndrome; PPEDS, Palmar-plantar erythrodysesthesia syndrome.
Undifferentiated pleomorphic sarcoma
The UPS subtype has been included in almost all clinical trials that led to the approval of second line therapies. Anthracyclines, ifosfamide, gemcitabine and trabectedin are active drugs in this histology but UPS is primarily sensitive to the combination of doxorubicin and ifosfamide (86). Based on a phase II study, the combination of gemcitabine with docetaxel was considered to be active in UPS (83), but later a larger phase III study confirmed that the combination of epirubicin with ifosfamide was more effective (87). Despite the meager research of immunotherapy in STS, a small phase II study testing the anti-PD1 antibody pembrolizumab in UPS showed 40% objective response rate (88), placing pembrolizumab a potential therapeutic option for the future.
Synovial sarcoma
Although SS is particular sensitive to ifosfamide (89), high dose of ifosfamide is not superior to the combination of anthracycline and ifosfamide in the first line (87). After first-line chemotherapy, trabectedin monotherapy showed better responses for SS compared to other STS subtypes (90), as well as the TKI pazopanib (85).
Research on new targeted agents has led to the exploration of potential molecular targets. In SS, the specific gene fusion SYT-SXX leads to the hyperexpression of intracellular pathways involved in survival and metastases, highlighting a number of potential targets (91). The oncogenic fusion SYT-SXX results in SMARCB1/INI1 proteolytic degradation, boosting the action of EZH2 on heterochromatin (92). Tazemetostat, an EZH2-inhibitor, has shown activity in preliminary results of a phase 1/2 clinical trial (NCT02601950) (93) and a phase 2 trial (NCT02601950) is currently ongoing.
Epithelioid sarcoma
Due to its rarity, prospective data on effectiveness of chemotherapeutic agents in ES are scarce. Retrospective analyses showed that gemcitabine-based and anthracycline-based chemotherapy regimens are active in metastatic ES (94). The combination gemcitabine/docetaxel is thus a second-line option for these patients. Trabectedin has been reported ineffective in ES (95), and pazopanib showed an inferior PFS and OS when compared to anthracyclines and to gemcitabine in a retrospective study (81).
ES is marked by SMARCB1/INI1 deficiency in a 90% of cases, and patients with this histology are included in the previously detailed clinical trials with tazemetostat (NCT02601950; NCT02601950).
Clear cell sarcoma
The CCS is a rare entity without prospective clinical trials and it is considered a primarily chemo-resistant sarcoma (81).
No objective responses have been reported with pazopanib, and only a small case series reporting partial responses with sorafenib and sunitinib has been reported (96).
Tinvatinib, a MET-inhibitor, has been tested in a phase II trial with 11 cases where a clinical benefit rate of 36% and a median PFS of 1.9 months were documented (97). The MET/ALK-inhibitor crizotinib and several immunotherapeutic options have also been tested in CSS patients without objective responses (98,99).
7. Discussion
STS of the upper extremity represent less than 10% of all STS and affect young patients, with a mean age at diagnosis of 38 years. Surgery remains the cornerstone of the treatment of STS of the upper extremities, which tend to be small and superficial, leading to a higher number of unplanned resections. This may be the cause of a higher rate of relapse of STS of this location. Besides, the anatomical particularities represent a surgical challenge, as wide excision may worsen the functional outcomes and debilitate this young patient subgroup. Although amputation could still be an obligatory option for selected cases, preservation of as many anatomical structures as possible performing a limb-sparing surgery (LSS) is desirable and represents the standard of care, using different reconstruction techniques to safeguard member's functionality.
However, about half of the patients, most commonly those with poor prognostic factors, who undergo surgery will relapse within the first 5 years following the intervention.
Adjuvant external-beam radiation therapy (RT) achieves similar outcomes as amputation when combined with LSS. Nowadays, different RT methods are available with less toxicity and similar efficacy but restricted to centers with expertise in the field. Whether administration of RT is preferable before or after surgery is an unanswered question. There are no great differences in terms of effectiveness and preoperative RT is associated with a higher rate of acute toxicity, but it may be preferred when downsizing of the tumor is required for increasing the probabilities of a successful LSS.
Adjuvant chemotherapy improves the survival of selected patients with STS of the extremities. However, clinical trials for adjuvant chemotherapy lack of specificity as their design have not considered the intrinsic heterogeneity of the disease and the prognostic factors due to the histological rarity and molecular heterogeneity of the subtypes. Given the greater incidence of high-grade STS in the upper extremities, it is expected that most patients with this diagnosis will undergo adjuvant chemotherapy.
Metastatic disease must be radically treated with surgery or radiotherapy whenever it is possible, as this practice may achieve an improvement in overall survival. Systemic therapy improves survival when radical therapy cannot be accomplished. The understanding of the underlying biology of each subtype of sarcoma is essential for the selection of one or another drug in each line of treatment.
With the information gathered above, the authors propose a therapeutic algorithm for the treatment of STS of the upper extremities according to the absence (Fig. 1) or presence of poor prognostic factors (Fig. 2) in the early disease stage or metastatic disease (Fig. 3). However, each individual patient should be discussed in the context of a specialized multidisciplinary meeting at the earliest possible stage of diagnosis, in order to establish a radical therapeutic plan, maximizing the profitability of the procedures and shortening the time between interventions. Indeed, the presence of a dedicated tumor board has been associated with an improvement of about 5% in the 2-year disease-free survival, and its absence has been defined as a new poor-prognostic factor for STS (29).
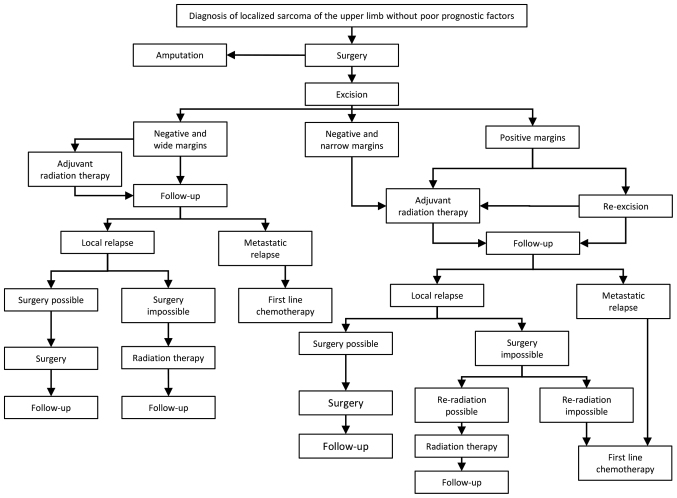
Figure 1.
Therapeutic algorithm of the localized STS of the upper extremities without poor prognostic factors. The poor prognostic factors included, high histological grade, size >5 cm, deep location and positive surgical margin status. STS, soft tissue sarcomas.
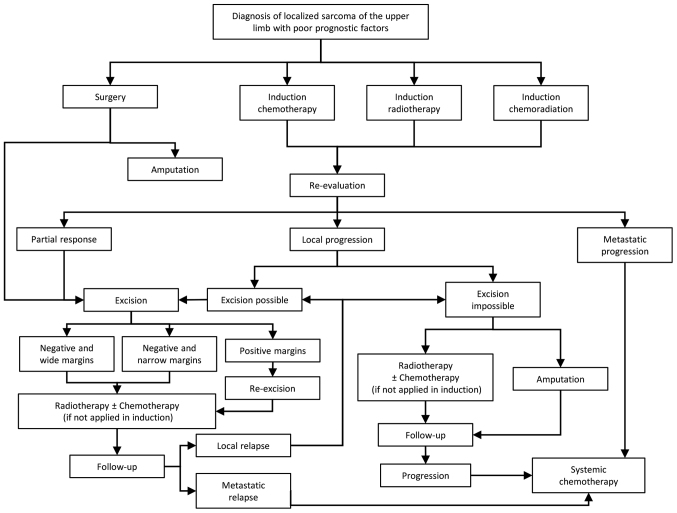
Figure 2.
Therapeutic algorithm of the localized STS of the upper extremities with poor prognostic factors. The poor prognostic factors included, high histological grade, size >5 cm, deep location and positive surgical margin status. STS, soft tissue sarcomas.
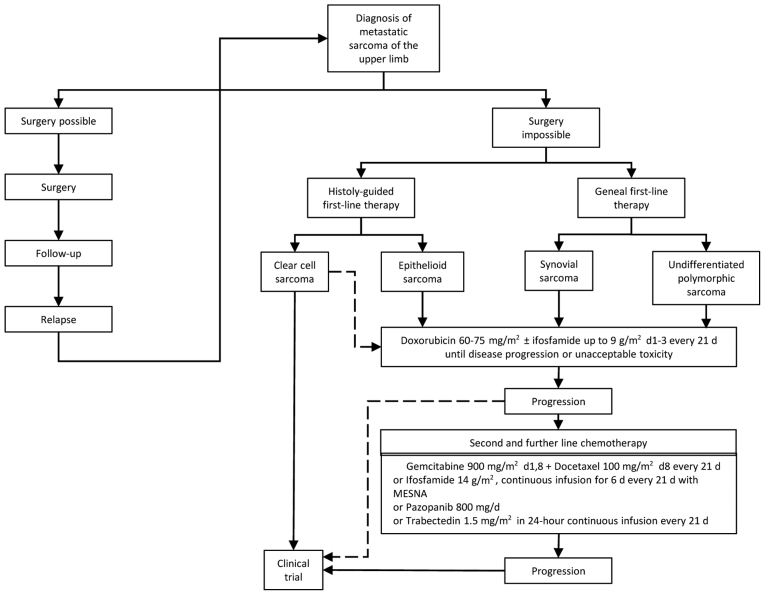
Figure 3.
Therapeutic algorithm of the metastatic STS of the upper extremities. STS, soft tissue sarcomas.
STS of the upper extremities represent a challenge due to anatomical and histopathological particularities, as well as to their low incidence. Although multidisciplinary treatments have increased the functional outcomes and the survival of these patients, a need of improvement of treatment for metastatic disease is of outmost importance, urging for multinational cooperation for the recruitment of patients in multicenter clinical trials.
Acknowledgements
Not applicable.
Funding
Funding was received from the Hellenic Study group of psychoneuroimmunology in cancer.
Availability of data and materials
Not applicable.
Authors' contributions
AK and VK conceived and designed the review. JDM performed the literature review. All authors were involved in the preparation and revision of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, Casali PG, RARECARE Working Group Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur J Cancer. 2013;49:684–695. doi: 10.1016/j.ejca.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of Sarcoma. Clin Sarcoma Res. 2012;2:14. doi: 10.1186/2045-3329-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafson P, Arner M. Soft tissue sarcoma of the upper extremity: Descriptive data and outcome in a population-based series of 108 adult patients. J Hand Surg Am. 1999;24:668–674. doi: 10.1053/jhsu.1999.0668. [DOI] [PubMed] [Google Scholar]
- 4.Gerrand CH, Wunder JS, Kandel RA, O'Sullivan B, Catton CN, Bell RS, Bell RS, Griffin AM, Davis AM. The influence of anatomic location on functional outcome in lower-extremity soft-tissue sarcoma. Ann Surg Oncol. 2004;11:476–482. doi: 10.1245/ASO.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Müller DA, Beltrami G, Scoccianti G, Frenos F, Capanna R. Combining limb-sparing surgery with radiation therapy in high-grade soft tissue sarcoma of extremities-is it effective? Eur J Surg Oncol. 2016;42:1057–1063. doi: 10.1016/j.ejso.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone, IARC Press. 2002. World Health Organization Classification of Tumours; p. Lyon. [Google Scholar]
- 7.Lazerges C. Soft tissue sarcomas of the forearm, wrist and hand. Hand Surg Rehabil. 2017;36:233–243. doi: 10.1016/j.hansur.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Strauss DC, Qureshi YA, Hayes AJ, Thway K, Fisher C, Thomas JM. The role of core needle biopsy in the diagnosis of suspected soft tissue tumours. J Surg Oncol. 2010;102:523–529. doi: 10.1002/jso.21600. [DOI] [PubMed] [Google Scholar]
- 9.Korah MP, Deyrup AT, Monson DK, Oskouei SV, Weiss SW, Landry J, Godette KD. Anatomic tumor location influences the success of contemporary limb-sparing surgery and radiation among adults with soft tissue sarcomas of the extremities. Int J Radiat Oncol Biol Phys. 2012;82:933–939. doi: 10.1016/j.ijrobp.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Dailiana ZH, Bougioukli S, Varitimidis S, Kontogeorgakos V, Togia E, Vlychou M, Malizos KN. Tumors and tumor-like lesions mimicking carpal tunnel syndrome. Arch Orthop Trauma Surg. 2014;134:139–144. doi: 10.1007/s00402-013-1901-8. [DOI] [PubMed] [Google Scholar]
- 11.Nandra R, Forsberg J, Grimer R. If your lump is bigger than a golf ball and growing, think Sarcoma. Eur J Surg Oncol. 2015;41:1400–1405. doi: 10.1016/j.ejso.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Callister MD, Ballo MT, Pisters PW, Patel SR, Feig BW, Pollock RE, Benjamin RS, Zagars GK. Epithelioid sarcoma: Results of conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:384–391. doi: 10.1016/S0360-3016(01)01646-7. [DOI] [PubMed] [Google Scholar]
- 13.Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, Tsuchiya H, Beppu Y, Morioka H, Yabe H, Japanese Musculoskeletal Oncology Group Clear cell sarcoma of tendons and aponeuroses: A study of 75 patients. Cancer. 2007;109:109–116. doi: 10.1002/cncr.22380. [DOI] [PubMed] [Google Scholar]
- 14.Walker EA, Salesky JS, Fenton ME, Murphey MD. Magnetic resonance imaging of malignant soft tissue neoplasms in the adult. Radiol Clin North Am. 2011;49(vi):1219–1234. doi: 10.1016/j.rcl.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: Distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224:99–104. doi: 10.1148/radiol.2241011113. [DOI] [PubMed] [Google Scholar]
- 16.Vasileios KA, Eward WC, Brigman BE. Surgical treatment and prognosis in patients with high-grade soft tissue malignant fibrous histiocytoma of the extremities. Arch Orthop Trauma Surg. 2012;132:955–961. doi: 10.1007/s00402-012-1510-y. [DOI] [PubMed] [Google Scholar]
- 17.Heslin MJ, Lewis JJ, Woodruff JM, Brennan MF. Core needle biopsy for diagnosis of extremity soft tissue sarcoma. Ann Surg Oncol. 1997;4:425–431. doi: 10.1007/BF02305557. [DOI] [PubMed] [Google Scholar]
- 18.Errani C, Traina F, Perna F, Calamelli C, Faldini C. Current concepts in the biopsy of musculoskeletal tumors. ScientificWorldJournal. 2013;013:538152. doi: 10.1155/2013/538152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo M, Pressney I, Hargunani R, Saifuddin A. Small, superficial, indeterminate soft-tissue lesions as suspected sarcomas: Is primary excision biopsy suitable? Skeletal Radiol. 2017;46:919–924. doi: 10.1007/s00256-017-2635-4. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijden L, Gibbons CL, Hassan AB, Kroep JR, Gelderblom H, van Rijswijk CS, Nout RA, Bradley KM, Athanasou NA, Dijkstra PD, et al. A multidisciplinary approach to giant cell tumors of tendon sheath and synovium-a critical appraisal of literature and treatment proposal. J Surg Oncol. 2013;107:433–445. doi: 10.1002/jso.23220. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol. 1985;3:1344–1348. doi: 10.1200/JCO.1985.3.10.1344. [DOI] [PubMed] [Google Scholar]
- 22.Puhaindran ME, Rothrock CP, Athanasian EA. Surgical management for malignant tumors of the thumb. Hand (NY) 2011;6:373–377. doi: 10.1007/s11552-011-9349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv268–iv269. doi: 10.1093/annonc/mdy095. [DOI] [PubMed] [Google Scholar]
- 24.Serpell JW, Ball AB, Robinson MH, Fryatt I, Fisher C, Thomas JM. Factors influencing local recurrence and survival in patients with soft tissue sarcoma of the upper limb. Br J Surg. 1991;78:1368–1372. doi: 10.1002/bjs.1800781132. [DOI] [PubMed] [Google Scholar]
- 25.Lin PP, Guzel VB, Pisters PWT, Zagars GK, Weber KL, Feig BW, Pollock RE, Yasko AW. Surgical management of soft tissue sarcomas of the hand and foot. Cancer. 2002;95:852–861. doi: 10.1002/cncr.10750. [DOI] [PubMed] [Google Scholar]
- 26.Perloff M, Holland JF. Surgical adjuvant chemotherapy. Annu Rev Med. 1977;28:475–488. doi: 10.1146/annurev.me.28.020177.002355. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Kent H, Costa J, Webber BL, Young R, Chabner B, Baker AR, Brennan MF, Chretien PB, Cohen MH, et al. Prospective randomized evaluation of the role of limb-sparing surgery, radiation therapy, and adjuvant chemoimmunotherapy in the treatment of adult soft-tissue sarcomas. Surgery. 1978;84:62–69. [PubMed] [Google Scholar]
- 28.Alamanda VK, Crosby SN, Archer KR, Song Y, Schwartz HS, Holt GE. Amputation for extremity soft tissue sarcoma does not increase overall survival: A retrospective cohort study. Eur J Surg Oncol. 2012;38:1178–1183. doi: 10.1016/j.ejso.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Blay JY, Soibinet P, Penel N, Bompas E, Duffaud F, Stoeckle E, Mir O, Adam J, Chevreau C, Bonvalot S, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28:2852–2859. doi: 10.1093/annonc/mdx484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantin J, McNeer GP, Chu FC, Booher RJ. The problem of local recurrence after treatment of soft tissue sarcoma. Ann Surg. 1968;168:47–53. doi: 10.1097/00000658-196807000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980:106–120. [PubMed] [Google Scholar]
- 32.Harati K, Goertz O, Pieper A, Daigeler A, Joneidi-Jafari H, Niggemann H, Stricker I, Lehnhardt M. Soft Tissue sarcomas of the extremities: Surgical margins can be close as long as the resected tumor has no ink on it. Oncologist. 2017;22:1400–1410. doi: 10.1634/theoncologist.2016-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradhan A, Cheung YC, Grimer RJ, Peake D, Al-Muderis OA, Thomas JM, Smith M. Soft-tissue sarcomas of the hand: Oncological outcome and prognostic factors. J Bone Joint Surg Br. 2008;90:209–214. doi: 10.1302/0301-620X.90B2.19601. [DOI] [PubMed] [Google Scholar]
- 34.Puhaindran ME, Healey JH, Athanasian EA. Single ray amputation for tumors of the hand. Clin Orthop Relat Res. 2010;468:1390–1395. doi: 10.1007/s11999-010-1389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puhaindran ME, Athanasian EA. Double ray amputation for tumors of the hand. Clin Orthop Relat Res. 2010;468:2976–2979. doi: 10.1007/s11999-010-1389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukushi S, Nishida Y, Urakawa H, Arai E, Kozawa E, Ishiguro N. Planned preservation surgery for soft tissue sarcomas adjacent to critical structures. Arch Orthop Trauma Surg. 2013;133:481–486. doi: 10.1007/s00402-013-1690-0. [DOI] [PubMed] [Google Scholar]
- 37.Clarkson PW, Griffin AM, Cotton CN, O'Sullivan B, Ferguson PC, Wunder JS, Bell RS. Epineural dissection is a safe technique that facilitates limb salvage surgery. Clin Orthop Relat Res. 2005;438:92–96. doi: 10.1097/01.blo.0000180057.22712.53. [DOI] [PubMed] [Google Scholar]
- 38.Radaelli S, Fiore M, Colombo C, Ford S, Palassini E, Sanfilippo R, Stacchiotti S, Sangalli C, Morosi C, Casali PG, Gronchi A. Vascular resection en-bloc with tumor removal and graft reconstruction is safe and effective in soft tissue sarcoma (STS) of the extremities and retroperitoneum. Surg Oncol. 2016;25:125–131. doi: 10.1016/j.suronc.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Elias DA, White LM, Simpson DJ, Kandel RA, Tomlinson G, Bell RS, Wunder JS. Osseous invasion by soft-tissue sarcoma: Assessment with MR imaging. Radiology. 2003;229:145–152. doi: 10.1148/radiol.2291020377. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson PC, Griffin AM, O'Sullivan B, Catton CN, Davis AM, Murji A, Bell RS, Wunder JS. Bone invasion in extremity soft-tissue sarcoma: Impact on disease outcomes. Cancer. 2006;106:2692–2700. doi: 10.1002/cncr.21949. [DOI] [PubMed] [Google Scholar]
- 41.Lin PP, Pino ED, Normand AN, Deavers MT, Cannon CP, Ballo MT, Pisters PW, Pollock RE, Lewis VO, Zagars GK, Yasko AW. Periosteal margin in soft-tissue sarcoma. Cancer. 2007;109:598–602. doi: 10.1002/cncr.22429. [DOI] [PubMed] [Google Scholar]
- 42.Megerle K, Sauerbier M. Reconstructive treatment of soft tissue sarcoma of the upper extremity. J Hand Surg Am. 2011;36:1241–1247. doi: 10.1016/j.jhsa.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Tseng JF, Ballo MT, Langstein HN, Wayne JD, Cormier JN, Hunt KK, Feig BW, Yasko AW, Lewis VO, Lin PP, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13:1209–1215. doi: 10.1245/s10434-006-9028-6. [DOI] [PubMed] [Google Scholar]
- 44.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–2725. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Stojadinovic A, Leung DHY, Allen P, Lewis JJ, Jaques DP, Brennan MF. Primary adult soft tissue sarcoma: Time-dependent influence of prognostic variables. J Clin Oncol. 2002;20:4344–4352. doi: 10.1200/JCO.2002.07.154. [DOI] [PubMed] [Google Scholar]
- 46.Italiano A, Delva F, Mathoulin-Pelissier S, Le Cesne A, Bonvalot S, Terrier P, Trassard M, Michels JJ, Blay JY, Coindre JM, Bui B. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: A multivariate analysis of the French sarcoma group database. Ann Oncol. 2010;21:2436–2441. doi: 10.1093/annonc/mdq238. [DOI] [PubMed] [Google Scholar]
- 47.Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P, Rosai J. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol. 2005;23:96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 48.Jebsen NL, Trovik CS, Bauer HC, Rydholm A, Monge OR, Hall KS, Alvegård T, Bruland OS. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: A Scandinavian sarcoma group study. Int J Radiat Oncol Biol Phys. 2008;71:1196–1203. doi: 10.1016/j.ijrobp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 50.Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: A SEER Analysis. Int J Radiat Oncol Biol Phys. 2010;77:203–209. doi: 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis AM, O'Sullivan B, Bell RS, Turcotte R, Catton CN, Wunder JS, Chabot P, Hammond A, Benk V, Isler M, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20:4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 52.Pisters PWT, Pollock RE, Lewis VO, Yasko AW, Cormier JN, Respondek PM, Feig BW, Hunt KK, Lin PP, Zagars G, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246:675–682. doi: 10.1097/SLA.0b013e318155a9ae. [DOI] [PubMed] [Google Scholar]
- 53.Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–347. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26:3440–3444. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 55.Folkert MR, Singer S, Brennan MF, Kuk D, Qin LX, Kobayashi WK, Crago AM, Alektiar KM. Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol. 2014;32:3236–3241. doi: 10.1200/JCO.2013.53.9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Wang S, Song Y, Liu X, Jin J, Wang W, Yu Z, Liu Y, Li Y. Postoperative intensity-modulated radiation therapy provides favorable local control and low toxicities in patients with soft tissue sarcomas in the extremities and trunk wall. Onco Targets Ther. 2015;8:2843–2847. doi: 10.2147/OTT.S88227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran QNH, Kim AC, Gottschalk AR, Wara WM, Phillips TL, O'Donnell RJ, Weinberg V, Haas-Kogan DA. Clinical outcomes of intraoperative radiation therapy for extremity sarcomas. Sarcoma. 2006;2006:91671. doi: 10.1155/SRCM/2006/91671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Call JA, Stafford SL, Petersen IA, Haddock MG. Use of intraoperative radiotherapy for upper-extremity soft-tissue sarcomas: Analysis of disease outcomes and toxicity. Am J Clin Oncol. 2014;37:81–85. doi: 10.1097/COC.0b013e31826b9b3d. [DOI] [PubMed] [Google Scholar]
- 59.Pellizzon ACA. Evidence and clinical outcomes of adult soft tissue sarcomas of the extremities treated with adjuvant high-dose-rate brachytherapy-a literature review. J Contemp Brachytherapy. 2014;6:318–22. doi: 10.5114/jcb.2014.45758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 61.Tierney JF, Mosseri V, Stewart LA, Souhami RL, Parmar MK. Adjuvant chemotherapy for soft-tissue sarcoma: Review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72:469–475. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 63.Woll PJ, Reichardt P, Le Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ, Leahy M, Van Coevorden F, Verweij J, Hogendoorn PC, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–1054. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 64.Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, Singer S. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–1672. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 65.Gronchi A, Frustaci S, Mercuri M, Martin J, Lopez-Pousa A, Verderio P, Mariani L, Valagussa P, Miceli R, Stacchiotti S, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: A randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30:850–856. doi: 10.1200/JCO.2011.37.7218. [DOI] [PubMed] [Google Scholar]
- 66.Frustaci S, Gherlinzoni F, De Paoli A, Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti G, Barbieri E, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 67.Gronchi A, Verderio P, De Paoli A, Ferraro A, Tendero O, Majó J, Martin J, Comandone A, Grignani G, Pizzamiglio S, et al. Quality of surgery and neoadjuvant combined therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and trunk wall. Ann Oncol. 2013;24:817–823. doi: 10.1093/annonc/mds501. [DOI] [PubMed] [Google Scholar]
- 68.Wray CJ, Benjamin RS, Hunt KK, Cormier JN, Ross MI, Feig BW. Isolated limb perfusion for unresectable extremity sarcoma: Results of 2 single-institution phase 2 trials. Cancer. 2011;117:3235–3241. doi: 10.1002/cncr.25850. [DOI] [PubMed] [Google Scholar]
- 69.Eggermont AM, ten Hagen TL. Isolated limb perfusion for extremity soft-tissue sarcomas, in-transit metastases, and other unresectable tumors: Credits, debits, and future perspectives. Curr Oncol Rep. 2001;3:359–367. doi: 10.1007/s11912-001-0090-8. [DOI] [PubMed] [Google Scholar]
- 70.Cheney MD, Giraud C, Goldberg SI, Rosenthal DI, Hornicek FJ, Choy E, Mullen JT, Chen YL, Delaney TF. MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol. 2014;109:593–596. doi: 10.1002/jso.23541. [DOI] [PubMed] [Google Scholar]
- 71.Rothermundt C, Whelan JS, Dileo P, Strauss SJ, Coleman J, Briggs TW, Haile SR, Seddon BM. What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer. 2014;110:2420–2426. doi: 10.1038/bjc.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abatzoglou S, Turcotte RE, Adoubali A, Isler MH, Roberge D. Local recurrence after initial multidisciplinary management of soft tissue sarcoma: Is there a way out? Clin Orthop Relat Res. 2010;468:3012–3018. doi: 10.1007/s11999-010-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potter DA, Glenn J, Kinsella T, Glatstein E, Lack EE, Restrepo C, White DE, Seipp CA, Wesley R, Rosenberg SA. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. 1985;3:353–366. doi: 10.1200/JCO.1985.3.3.353. [DOI] [PubMed] [Google Scholar]
- 74.Marulli G, Mammana M, Comacchio G, Rea F. Survival and prognostic factors following pulmonary metastasectomy for sarcoma. J Thorac Dis. 2017;9(Suppl 12):S1305–S1315. doi: 10.21037/jtd.2017.03.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith R, Pak Y, Kraybill W, Kane JM., III Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol. 2009;35:356–3561. doi: 10.1016/j.ejso.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Borden EC, Amato DA, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, Lerner HJ, Carbone PP. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol. 1987;5:840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 77.Edmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S, Frytak S, Parkinson DR. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 78.Antman K, Crowley J, Balcerzak SP, Rivkin SE, Weiss GR, Elias A, Natale RB, Cooper RM, Barlogie B, Trump DL, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276–1285. doi: 10.1200/JCO.1993.11.7.1276. [DOI] [PubMed] [Google Scholar]
- 79.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan J, Hohenberger P, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 80.Lilly Reports Results of Phase 3 Soft Tissue Sarcoma Study of LARTRUVO®, corp-author. https://lilly.mediaroom.com/index.php?s=9042&item=137861. [Jan 18;2019 ];
- 81.Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: What is standard, what is new. BMC Med. 2017;15:109. doi: 10.1186/s12916-017-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demetri GD, Von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K, Tawbi H, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, Fanucchi M, Harmon DC, Schuetze SM, Reinke D, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 84.García-del-Muro X, López-Pousa A, Maurel J, Martín J, Martínez-Trufero J, Casado A, Gómez-España A, Fra J, Cruz J, Poveda A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish group for research on sarcomas study. J Clin Oncol. 2011;29:2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]
- 85.Van Der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 86.Young RJ, Litière S, Lia M, Hogendoorn PCW, Fisher C, Mechtersheimer G, Daugaard S, Sciot R, Collin F, Messiou C, et al. Predictive and prognostic factors associated with soft tissue sarcoma response to chemotherapy: A subgroup analysis of the European Organisation for research and treatment of cancer 62012 study. Acta Oncol. 2017;56:1013–1020. doi: 10.1080/0284186X.2017.1315173. [DOI] [PubMed] [Google Scholar]
- 87.Gronchi A, Ferrari S, Quagliuolo V, Broto JM, Pousa AL, Grignani G, Basso U, Blay JY, Tendero O, Beveridge RD, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18:812–822. doi: 10.1016/S1470-2045(17)30334-0. [DOI] [PubMed] [Google Scholar]
- 88.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SH, Chang MH, Baek KK, Han B, Lim T, Lee J, Park JO. High-dose ifosfamide as second- or third-line chemotherapy in refractory bone and soft tissue sarcoma patients. Oncology. 2011;80:257–261. doi: 10.1159/000328795. [DOI] [PubMed] [Google Scholar]
- 90.Kawai A, Araki N, Sugiura H, Ueda T, Yonemoto T, Takahashi M, Morioka H, Hiraga H, Hiruma T, Kunisada T, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015;16:406–416. doi: 10.1016/S1470-2045(15)70098-7. [DOI] [PubMed] [Google Scholar]
- 91.de Necochea-Campion R, Zuckerman LM, Mirshahidi HR, Khosrowpour S, Chen CS, Mirshahidi S. Metastatic biomarkers in synovial sarcoma. Biomark Res. 2017;5:4. doi: 10.1186/s40364-017-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kadoch C, Crabtree GR. Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell. 2013;153:71–85. doi: 10.1016/j.cell.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agulnik M, Tannir NM, Pressey JG, Gounder MM, Cote GM, Roche M, Doleman S, Blakemore SJ, Clawson A, Daigle S, et al. A phase II, multicenter study of the EZH2 inhibitor tazemetostat in adult subjects with INI1-negative tumors or relapsed/refractory synovial sarcoma. J Clin Oncol. 2016;34:11071. doi: 10.1200/JCO.2016.34.15_suppl.TPS11071. [DOI] [Google Scholar]
- 94.Pink D, Richter S, Gerdes S, Andreou D, Tunn PU, Busemann C, Ehninger G, Reichardt P, Schuler MK. Gemcitabine and docetaxel for epithelioid sarcoma: Results from a retrospective, multi-institutional analysis. Oncology. 2014;87:95–103. doi: 10.1159/000362602. [DOI] [PubMed] [Google Scholar]
- 95.Grivas A, Trafalis DT, Thanopoulou E, Ziras NG, Athanasiou AE. Treatment with trabectedin: Should be indicated to all soft tissue sarcoma histotypes? J BUON. 2010;15:791–793. [PubMed] [Google Scholar]
- 96.Cornillie J, van Cann T, Wozniak A, Hompes D, Schöffski P. Biology and management of clear cell sarcoma: State of the art and future perspectives. Expert Rev Anticancer Ther. 2016;16:839–845. doi: 10.1080/14737140.2016.1197122. [DOI] [PubMed] [Google Scholar]
- 97.Wagner AJ, Goldberg JM, Dubois SG, Choy E, Lee R, Pappo A, Geller J, Judson I, Hogg D, Senzer N, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: Results of a multicenter phase 2 trial. Cancer. 2012;118:5894–5902. doi: 10.1002/cncr.27582. [DOI] [PubMed] [Google Scholar]
- 98.Schöffski P, Adkins D, Blay JY, Gil T, Elias AD, Rutkowski P, Pennock GK, Youssoufian H, Gelderblom H, Willey R, Grebennik DO. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer. 2013;49:3219–3228. doi: 10.1016/j.ejca.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 99.Goldberg JM, Fisher DE, Demetri GD, Neuberg D, Allsop SA, Fonseca C, Nakazaki Y, Nemer D, Raut CP, George S, et al. Biologic activity of autologous, granulocyte-macrophage colony-stimulating factor secreting alveolar soft-part sarcoma and clear cell sarcoma vaccines. Clin Cancer Res. 2015;21:3178–3186. doi: 10.1158/1078-0432.CCR-14-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.