Keywords: nerve regeneration, stroke, cerebral ischemia, ischemic stroke, peroxisome proliferator-activated receptor γ, single-nucleotide polymorphism, haplotype analysis, interaction, case-control study, Chinese Han population, neural regeneration
Abstract
Two common polymorphisms of the peroxisome proliferator-activated receptor gamma (PPARG) gene, rs1801282 and rs3856806, may be important candidate gene loci affecting the susceptibility to ischemic stroke. This case-control study sought to identify the relationship between these two single-nucleotide polymorphisms and ischemic stroke risk in a northern Chinese Han population. A total of 910 ischemic stroke participants were recruited from the First Hospital of China Medical University, Shenyang, China as a case group, of whom 895 completed the study. The 883 healthy controls were recruited from the Health Check Center of the First Hospital of China Medical University, Shenyang, China. All participants or family members provided informed consent. The study protocol was approved by the Ethics Committee of the First Hospital of China Medical University, China on February 20, 2012 (approval No. 2012-38-1). The protocol was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-COC-17013559). Plasma genomic DNA was extracted from all participants and analyzed for rs1801282 and rs3856806 single nucleotide polymorphisms using a SNaPshot Multiplex sequencing assay. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression to estimate the association between ischemic stroke and a particular genotype. Results demonstrated that the G allele frequency of the PPARG gene rs1801282 locus was significantly higher in the case group than in the control group (P < 0.001). Individuals carrying the G allele had a 1.844 fold increased risk of ischemic stroke (OR = 1.844, 95% CI: 1.286–2.645, P < 0.001). Individuals carrying the rs3856806 T allele had a 1.366 fold increased risk of ischemic stroke (OR = 1.366, 95% CI: 1.077–1.733, P = 0.010). The distribution frequencies of the PPARG gene haplotypes rs1801282-rs3856806 in the control and case groups were determined. The frequency of distribution in the G-T haplotype case group was significantly higher than that in the control group. The risk of ischemic stroke increased to 2.953 times in individuals carrying the G-T haplotype (OR = 2.953, 95% CI: 2.082–4.190, P < 0.001). The rs1801282 G allele and rs3856806 T allele had a multiplicative interaction (OR = 3.404, 95% CI: 1.631–7.102, P < 0.001) and additive interaction (RERI = 41.705, 95% CI: 14.586–68.824, AP = 0.860; 95% CI: 0.779–0.940; S = 8.170, 95% CI: 3.772–17.697) on ischemic stroke risk, showing a synergistic effect. Of all ischemic stroke cases, 86% were attributed to the interaction of the G allele of rs1801282 and the T allele of rs3856806. The effect of the PPARG rs1801282 G allele on ischemic stroke risk was enhanced in the presence of the rs3856806 T allele (OR = 8.001 vs. 1.844). The effect of the rs3856806 T allele on ischemic stroke risk was also enhanced in the presence of the rs1801282 G allele (OR = 2.546 vs. 1.366). Our results confirmed that the G allele of the PPARG gene rs1801282 locus and the T allele of the rs3856806 locus may be independent risk factors for ischemic stroke in the Han population of northern China, with a synergistic effect between the two alleles.
Chinese Library Classification No. R446; R741
Introduction
Worldwide, stroke is the main cause of disability and death (Powers et al., 2015; Salinas et al., 2016; Lee et al., 2017; Gao et al., 2018; Liska et al., 2018). In China, the incidence of stroke exhibits substantial geographic variation: Northern China has the highest incidence of stroke, whereas Southern China has a significantly lower incidence (Peng et al., 2014; Wang et al., 2017a; Sun et al., 2018). Ischemic stroke is a multifactorial and multigenic disease. Identifying genetic variants associated with ischemic stroke risk could shed light on the pathogenesis of stroke and lead to new approaches for the management and prevention of this complicated disease. Although several genome-wide association studies have assessed various polymorphisms that may contribute to ischemic stroke (Deng et al., 2010; Liu et al., 2011; Ranta and Lichtman, 2015; Wang et al., 2016b, 2017b), the genetic variants correlated with the predisposition to ischemic stroke have not been unequivocally determined in Chinese individuals.
Peroxisome proliferator-activated receptor gamma (PPARG) is a protective factor against the pathogenesis of ischemic stroke and is involved in inhibiting the pathogenesis and progression of hypertension, type 2 diabetes, hyperlipidemia, metabolic syndromes, obesity, and some inflammatory diseases (Ivanova et al., 2015; Liu and Wang, 2015; Liu et al., 2015, 2018; Yuan et al., 2015; Lee et al., 2017; Lv et al., 2017; Kim et al., 2018). A number of single-nucleotide polymorphisms (SNPs) in the PPARG gene have gained increasing attention, and influence the expression or activity of PPARG (Janani and Ranjitha Kumari, 2015). Among these SNPs, two common polymorphisms of the PPARG gene, rs3856806 (His477His, also known as C1431T or C161T) and rs1801282 (Pro12Ala), have been extensively researched in terms of their associations with atherosclerosis. There are reports of positive associations between increased risk and polymorphisms of the PPARγ gene; however, some reports have suggested a lower risk of atherosclerosis in carriers (Wu et al., 2013; Wang et al., 2015; Wei et al., 2016). Furthermore, single-locus independent main effects could explain only a part of the observed heritability – a phenomenon often referred to as the ‘‘missing heritability’’ problem (Maher, 2008). Therefore, studying combined SNP-SNP effects can improve the interpretation of genetic information (Prabhu and Pe’er, 2012). Therefore, SNP-SNP interactions and their departure from independent effects, which is identified by epistasis in genetics and epidemiological effect modification, could improve knowledge of the hereditary component of ischemic stroke.
Therefore, we aimed to assess the association between PPARG genetic polymorphisms at the rs1801282 and rs3856806 loci and ischemic stroke risk, and the interactions between the two loci in multiplicative and additive models.
Participants and Methods
Design
A case-control study.
Subjects
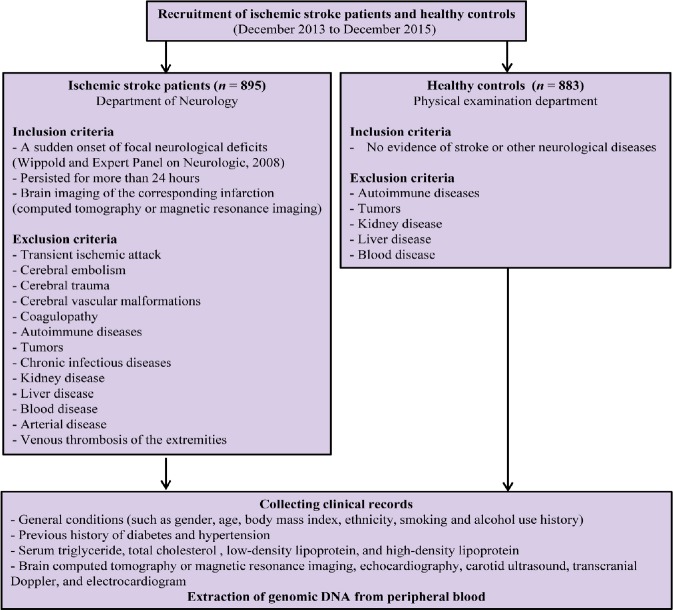
Our sample included 895 ischemic stroke patients and 883 age- and sex-matched healthy controls. Participant data were collected from December 2013 to December 2015, from the First Hospital of China Medical University, Shenyang, China. The criteria for the inclusion and exclusion of patients and controls are in the study flow chart (Figure 1).
Figure 1.
Study flow chart showing the inclusion and exclusion criteria of patients and controls.
All patients were between 40 and 80 years of age. If the patients are first diagnosed with an acute ischemic stroke based on neurological examination and radiography, the patient was determined to be eligible. None of the controls showed evidence of stroke or other neurological diseases. Patients with transient ischemic attack, cerebral embolism, cerebral trauma, cerebral vascular malformations, coagulopathy, autoimmune diseases, tumors, or chronic infectious diseases were excluded. Cases of kidney or liver disease, blood disease, occlusive arterial disease or venous thrombosis of the extremities were also excluded. All participants were of the Han nationality and lived in Liaoning province in northern China. Body mass index was classified according to the Asian obesity classification; body mass index > 22.9 kg/m2 was considered overweight (Jih et al., 2014). Hyperlipidemia was defined as a total plasma cholesterol level of 5.72 mM, a plasma triglyceride level of 1.7 mM (Wang et al., 2011), or if lipid-lowering drugs were currently in use. This study was approved by The Institutional Ethical Committee of the First Hospital of China Medical University on February 20, 2012 (approval No. 2012-38-1; Additional file 1 (237KB, pdf) ). This study was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants signed written informed consent. The protocol was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-COC-17013559).
SNP selection
Well-studied or functional SNPs were selected in view of previous studies that recorded associations between SNPs in PPARG and atherosclerotic diseases (Wu et al., 2013; Wang et al., 2015; Wei et al., 2016). SNP ID numbers and detailed sequence information for rs1801282 and rs3856806 were acquired from the public dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/). The minor allele frequency of the above-mentioned two SNPs was greater than 0.05 in the Chinese Han population.
DNA extraction and genotyping
Genomic DNA was extracted and the SNaPshot reaction was performed for genotyping as previously described (Wang et al., 2016, 2017) using the SNaPshot Multiplex Kit (Applied Biosystems Co., Ltd., Foster City, CA, USA). Table 1 shows the sequences of the PCR primers. Experimental results were analyzed using an ABI 3130XL DNA sequence detector and GeneMapper 4.0 (Applied Biosystems Co., Ltd.).
Table 1.
Primer sequences
| SNP | Primer sequence | Product size (bp) |
|---|---|---|
| rs1801282 | Forward: 5′ CAG CCA ATT CAA GCC CAG | 296 |
| TCC T 3′ | ||
| Reverse: 5′ CCG TAT CTG GAA GGA ACT | ||
| TTA CCT TGT 3′ | ||
| rs3856806 | Forward: 5′ GCC AAG CTG CTC CAG AAA | 161 |
| ATG A 3′ | ||
| Reverse: 5′ TGG CAG TGG CTC AGG ACT | ||
| CTC T 3′ |
SNP: Single-nucleotide polymorphism.
Statistical analysis
Unless otherwise noted, all statistical analyses were performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). All tests were two-tailed, and significance was defined as P < 0.05. Pearson’s chi-square test was used to compare the distribution of demographic variables and examine differences between risk factors and genotypes for alleles and haplotypes between patients and controls. A chi-square goodness-of-fit test was used for testing Hardy-Weinberg equilibrium for each genotype. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression to estimate the association between ischemic stroke and a particular genotype. The Quanto power calculator was used to calculate power in our cohort. Assuming a genotypic relative risk for a recessive model of 2, a minor allele frequency of 0.15, a 1.88% population prevalence of ischemic stroke, and a Type I error probability of 0.05 in the sample size of 895 patient samples and 883 healthy controls, we would be unable to reject the null hypothesis that OR = 1 with a power of 99.99%. Based on the observed frequencies of the two SNPs, the SHEsis analysis platform was used to calculate linkage disequilibrium indices (D’ and r2) and infer haplotype frequencies (Shi and He, 2005; Li et al., 2009).
The attributable proportion due to interaction (AP) and relative excess risk due to interaction (RERI) were used to test additive SNP-SNP interactions. If RERI and AP equal 0, there is no biologic interaction (Leng et al., 2016). Gene interactions between the two SNPs were evaluated under two models of statistical epistasis, the additive and multiplicative models, using a customized Microsoft Excel spreadsheet as described by Andersson et al. (2005). Confounding factors, including sex, age, body mass index, diabetes mellitus, hypertension, history of alcohol use, history of smoking, history of ischemic stroke, and hyperlipidemia, were controlled in additive interaction analysis.
Results
Number of subject analysis
To determine the potential associations of PPARG SNPs with the risk of ischemic stroke, 910 ischemic stroke patients and 883 controls were recruited, but only 895 patients completed the study (Figure 1): 9 patients were excluded for cardiogenic cerebral embolisms, 2 patients were excluded for cerebral arteritis, and 4 patients were excluded for unknown risk factors for ischemic stroke. All controls completed the study. This study collected final clinical data and peripheral blood specimens from 895 patients with ischemic stroke and 883 age- and sex-matched controls.
Clinical characteristics of study subjects
The summary of the general characteristics of the patient and control groups are shown in Table 2. There were no significant differences in sex (P = 0.091) or age (P = 0.179) between controls and patients. However, conventional risk factors for ischemic stroke, such as diabetes mellitus, body mass index, hyperlipidemia, hypertension, history of alcohol use, and history of smoking, were remarkably more prevalent in the patient group than in the control group.
Table 2.
Characteristics and risk factors for stroke
| Variable | Cases [n (%)] | Controls [n (%)] | P value |
|---|---|---|---|
| Age (≤ 60/> 60, years) | 319 (35.6)/ | 288 (32.6)/ | 0.179 |
| 576 (64.4) | 595 (67.4) | ||
| Sex (male/female) | 499 (55.8)/ | 457 (51.8)/ | 0.091 |
| 396 (44.2) | 426 (48.2) | ||
| BMI (≤ 22.9/> 22.9, kg/m2) | 454 (50.7)/ | 274 (65.0)/ | < 0.001 |
| 441 (49.3) | 148 (35.0) | ||
| Diabetes mellitus | 234 (26.1) | 64 (7.2) | < 0.001 |
| Hypertension | 552 (61.7) | 178 (20.2) | < 0.001 |
| History of smoking | 310 (34.6) | 137 (15.5) | < 0.001 |
| History of alcohol use | 154 (17.2) | 98 (11.1) | < 0.001 |
| History of ischemic stroke | 63 (7.0) | 46 (5.2) | 0.108 |
| Hyperlipidemia | 324 (36.2) | 177 (20.0) | < 0.001 |
Age, BMI, sex, hypertension, diabetes mellitus, history of alcohol use, history of smoking, history of ischemic stroke, and hyperlipidemia were assessed using Pearson’s chi-square test. There was no significant difference in age, sex and history of ischemic stroke between the patients and controls. BMI, hypertension, diabetes mellitus, history of alcohol use, history of smoking, and history of ischemic stroke and hyperlipidemia were associated with ischemic stroke. BMI: Body mass index.
Genotype analysis
All allele distributions were in Hardy-Weinberg equilibrium (rs1801282: P = 0.128; rs3856806: P = 0.959). The allele frequencies and genotypes of the two SNPs among the 895 patients with ischemic stroke and 883 control participants are shown in Table 3.
Table 3.
Allele and genotype frequencies of genetic polymorphisms among cases and controls and their main effects on stroke risk
| SNP | Cases | Percent | Controls | Percent | OR (95% CI) *a | P value b |
|---|---|---|---|---|---|---|
| rs1801282 | ||||||
| CC (reference) | 756 | 84.5 | 807 | 91.4 | 1.00 (reference) | |
| CG | 129 | 14.4 | 75 | 8.5 | 1.748 (1.209–2.581) | 0.003 |
| GG | 10 | 1.1 | 1 | 0.1 | 3.736 (0.430–32.436) | 0.232 |
| Dominant model CG + GG vs. CC | 1.844 (1.286–2.645) | 0.001 | ||||
| Recessive model CC + CG vs. GG | 0.298 (0.034–2.601) | 0.274 | ||||
| rs3856806 | ||||||
| CC (reference) | 515 | 57.5 | 587 | 66.5 | 1.00 (reference) | – |
| CT | 322 | 36.0 | 274 | 31.0 | 1.290 (1.011–1.647) | 0.041 |
| TT | 58 | 6.5 | 22 | 2.5 | 2.317 (1.274–4.214) | 0.006 |
| Dominant model CT + TT vs. CC | 1.366 (1.077–1.733) | 0.010 | ||||
| Recessive model CC + CT vs. TT | 0.424(0.239–0.751) | 0.003 |
ORs and 95% CIs were calculated based on multivariable logistic regression. Individuals with the heterozygous CG genotype for rs1801282 had a higher risk of ischemic stroke than individuals with the homozygous wild-type CC. The CG + GG genotype was associated with a significantly increased ischemic stroke risk. The CT and TT genotypes of rs3856806 were represented with increased frequency in the ischemic stroke patient group. The CT + TT genotype of rs3856806 was associated with ischemic stroke. *ORs and 95% CIs were calculated by logistic regression. a, bAdjusted OR (95% CI) and P value, adjusted for age, sex, body mass index, hypertension, diabetes mellitus, history of alcohol use, history of smoking, history of ischemic stroke, and hyperlipidemia. SNP: Single nucleotide polymorphism; OR: odds ratio; CI: confidence interval.
Compared with individuals with the homozygous wild-type CC genotype for rs1801282, individuals with the heterozygous CG genotype (14.4%) had an increased risk of ischemic stroke (adjusted OR = 1.748, 95% CI: 1.209–3.736, P = 0.003). In the dominant model, compared with the CC genotype of rs1801282, the CG + GG genotype was correlated with a significantly increased risk of ischemic stroke (adjusted OR = 1.844, 95% CI: 1.286–2.645, P < 0.001). The CT (36.0%) and TT (6.5%) rs3856806 genotypes were represented with increased frequency in the patients (adjusted OR = 1.290, 95% CI: 1.011–1.647, P = 0.041 and adjusted OR = 2.317, 95% CI: 1.247–4.214, P = 0.006, respectively). The CT + TT rs3856806 genotype was significantly associated with ischemic stroke (P = 0.010).
Haplotype analysis
The two SNPs were in linkage disequilibrium in our study population (i.e., D’ > 0.8 or r2 > 0.4). Of all possible haplotypes, only 3 had a frequency of > 0.03 among both patients and healthy controls and were included in the haplotype analysis. The haplotype analysis indicated that the frequency of the G-T haplotype of rs1801282-rs3856806 was significantly higher in patients than in healthy controls (OR = 2.953, 95% CI: 2.082–4.190, P < 0.001; Table 4).
Table 4.
Haplotype frequencies [n(%)] in cases and controls and their relationship to stroke risk
| Haplotype | Cases | Controls | OR (95% CI) | P value |
|---|---|---|---|---|
| C-C | 1329 (74.3) | 1415 (80.1) | 0.682 (0.580–0.803) | < 0.001 |
| C-T | 312 (17.4) | 274 (15.5) | 1.140 (0.954–1.362) | 0.148 |
| G-T | 126 (7.1) | 44 (2.5) | 2.953 (2.082–4.190) | < 0.001 |
ORs and 95% CIs were calculated using the SHEsis program. The frequency of the G-T haplotype of rs1801282-rs3856806 was significantly higher in patients than in controls. OR: Odds ratio; CI: confidence interval.
SNP-SNP interaction analysis
The dominant model was assessed in a further additive interaction analysis. The analysis below determined a significant interaction (RERI = 41.705, 95% CI: 14.586–68.824; AP = 0.860, 95% CI: 0.779, 0.940; S = 8.170, 95% CI: 3.772–17.697, P < 0.001) between rs1801282 and rs3856806 by controlling for age, sex, body mass index, diabetes mellitus, hypertension, history of alcohol use, history of smoking, history of ischemic stroke, and hyperlipidemia. Among all cases, those carrying the risk allele for both loci accounted for 86%.
The effect of the G risk allele at rs1801282 was modified by the T risk allele at rs3856806, as shown in Table 5, along with the results of each locus stratified by the other risk allele for ischemic stroke. The effect of the T risk allele at rs3856806 was stronger when rs1801282 had the G risk allele (adjusted OR = 8.001, 95% CI: 2.559–25.016, vs. adjusted OR = 1.188, 0.928–1.522), indicating synergistic epistasis (Table 5). A similar trend was detected for the effect of rs1801282 correlating with rs3856806 (adjusted OR = 2.546, 95% CI: 1.639–3.954, in the absence of rs3856806 vs. OR = 0.525, 95% CI: 0.245–1.123, when carrying both).
Table 5.
Stratified odds ratios for rs1801282 and rs3856806 with respect to the risk of ischemic stroke
| Stratum | SNPs | OR (95%CI) *a | P value b |
|---|---|---|---|
| Ischemic stroke | |||
| Stratified by rs1801282 | |||
| CG or GG | rs3856806 (CT or TT vs. CC) | 8.001 (2.559–25.016) | < 0.001 |
| CC | rs3856806 (CT or TT vs. CC) | 1.188 (0.928–1.522) | 0.171 |
| Stratified by rs3856806 | |||
| CT or TT | rs1801282 (CG or GG vs. CC) | 2.546 (1.639–3.954) | < 0.001 |
| CC | rs1801282 (CG or GG vs. CC) | 0.525 (0.245–1.123) | 0.097 |
ORs and 95% CIs were assessed based on multivariable logistic regression. The effect of the T risk allele at rs3856806 was stronger when rs1801282 had the G risk allele. A similar result was demonstrated for the effect of rs1801282 in relation to rs3856806. *ORs and 95% CIs were calculated by logistic regression. a, bAdjusted OR (95% CI) and P value, adjusted for age, sex, body mass index, hypertension, diabetes mellitus, history of alcohol use, history of smoking, history of ischemic stroke, and hyperlipidemia. SNP: Single nucleotide polymorphism; OR: odds ratio; CI: confidence interval.
Discussion
This study evaluated the association between two genetic polymorphisms of PPARG with ischemic stroke risk in a northern Chinese Han population of 895 cases and 883 controls. To our knowledge, this is the first study to suggest that two PPARG genetic variants, rs1801282 and rs3856806, play a considerable role in the etiology of ischemic stroke. Our study also revealed a synergistic SNP-SNP interaction between the two risk alleles. Our findings suggest that the PPARG rs1801282 CG genotype and rs3856806 CT/TT genotypes are associated with an increased risk of ischemic stroke.
PPARG is involved in controlling immune responses and preventing atherosclerosis. Additionally, activation of PPARG effectively attenuates neurodegeneration and inflammatory processes in the brain (Culman et al., 2007; Wang et al., 2016a; Liu et al., 2017; Wu et al., 2018; Xia et al., 2018; Li et al., 2019). Furthermore, neuronal PPARG deficiency increased oxidative stress and brain damage during middle cerebral artery occlusion in mice (Zhao et al., 2009). In combination with the recent observation that activation of retinoid X receptors (RXR)/PPARG is the basis for neuroprotection by bexarotene in ischemic stroke (Certo et al., 2015), these results support the notion that PPARG plays an important protective role against ischemic injury.
The common polymorphism rs1801282 (Pro12Ala) in the PPARG gene is associated with greater insulin sensitivity (Stumvoll et al., 2001; Moffett et al., 2005; Youssef et al., 2014). Because the PPARG-rs1801282 polymorphism likely reduces transcriptional activity (Stumvoll et al., 2001), it may reduce insulin signaling and suppress the function of pancreas islet B cells (Stefan et al., 2001), ultimately resulting in diabetes mellitus (Altshuler et al., 2000; Ye et al., 2014; Kasim et al., 2016; Priya et al., 2016). In recent years, the role of PPARG-rs1801282 expression has been identified to decrease lipoprotein lipase activity (Xie et al., 2014; Zhang et al., 2016) and influence triglyceride removal, leading to atherosclerosis and dyslipidemia. In the present study, using participants with the PPARG-rs1801282 CC genotype as the reference group, the CG + GG genotype had a 1.844-fold higher risk of ischemic stroke after logistic regression. Potentially, the effect of rs1801282 on ischemic stroke risk is, to some degree, mediated by an effect on glucose and lipid metabolism. However, previous findings regarding the association between PPARG-rs1801282 polymorphisms and ischemic stroke risk are inconsistent (Lee et al., 2006; Volcik et al., 2008). Genetic heterogeneity due to differences in ethnicity could contribute to these discrepancies. Further reasons for the varying results may be sample size, study design, or patient selection criteria. The precise mechanisms underlying these observations need further elucidation.
We demonstrated a significantly increased risk of ischemic stroke with high-risk genotypes, such as the PPARG-rs3856806 T allele. However, this polymorphism in exon 6 of the PPARG gene had a protective effect on atherosclerotic lipid profiles in previous studies (Wan et al., 2010; Yilmaz-Aydogan et al., 2011). PPARG-rs3856806 and reduced coronary artery disease risk were associated in patients in an Australian Caucasian cohort (Wang et al., 1999) and in Chinese patients (Wu et al., 2013). These studies placed emphasis on cardiogenic stroke. Distinctive mechanisms may be crucial in the development of acute ischemic coronary and cerebrovascular events (Cheng et al., 2012). This phenomenon might be explained at least in part by differences in sample selection and study design, as well as sample size. Although the functional significance of rs3856806 (His477His) is limited because it is a silent mutation, evidence determining the role of this polymorphism in the expression of PPARG by changing the efficiency of protein translation is scarce. Moreover, this polymorphism possibly participates in modification of PPARG activation, resulting in altered transcription levels of target genes in metabolic pathways (Meirhaeghe et al., 2000, 2005; Wu et al., 2013).
SNP-SNP interaction or statistical epistasis, as defined by Fisher in 1918, is hypothesized to explain part of the hereditary nature of the disease (Marchini et al., 2005; Evans et al., 2006). Furthermore, finding significant statistical links (epistatic or otherwise) between SNPs could provide a strong indication of the different molecular level interactions between cases and controls. Interestingly, through SNP-SNP interaction studies, we have identified a combination of two SNPs that contribute to a strong risk of ischemic stroke. SNP-SNP interactions were statistically significant under both models, and the additive model provided a better fit than the multiplicative model with respect to biological synergy. This suggests that these two loci are in linkage disequilibrium. We confirmed that the G-T haplotype of rs1801282-rs3856806 is a potential susceptibility marker for ischemic stroke in our Chinese Han population in northern China.
Some limitations should be considered when interpreting these results. First, as the participants of our analysis were limited to a Han Chinese population, whether these results can be extrapolated to other populations is uncertain. Nevertheless, potential confounding factors were minimized by matching age, ethnicity, and sex. Second, the current study lacked functional assessments through in vivo and in vitro experiments to identity whether the genetic variants suggested in our study modulate ischemic stroke risk through their influences on the expression or functions of their host genes. The most important limitation of this study was the sample size and the absence of large-scale investigations. Thus, some of these results could be attributed to chance.
In conclusion, two polymorphisms in PPARG, rs1801282 C>G and rs3856806 C>T, increase the susceptibility to ischemic stroke in a northern Chinese Han population. We further identified two SNPs that may play a combined role in ischemic stroke. Further study of the association between these two SNPs and ischemic stroke is necessary in larger studies. Moreover, the functional mechanisms by which these two SNPs act should be clearly elucidated to better understand their role in the etiology of ischemic stroke. Given that the disability caused by ischemic stroke increases socioeconomic burden, excellent strategies for prevention and for the early detection of high-risk populations is essential to address this global problem. Not only must individuals at risk be directed into disease surveillance networks, but we must also increase the likelihood that reperfusion therapy can be administered within the therapeutic window to provide benefit when a stroke occurs.
Additional files:
Additional file 1 (237KB, pdf) : Ethics document (Chinese)
Additional file 2: Open peer review reports 1 (108.3KB, pdf) and 2 (105.5KB, pdf) .
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81070913 (to ZYH). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study protocol was approved by the Ethics Committee of the First Hospital of China Medical University, China on February 20, 2012 (approval No. 2012-38-1). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the forms the participants or their family members have given their consent for the participants’ images and other clinical information to be reported in the journal. The participants or their family members understand that the participants’ names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of the First Hospital of China Medical University.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Sagar Gaikwad, Senior Research Fellow, Indian Institute of Advanced Research, India; Mercedes Fernandez, University of Bologna, Bologna, Italy.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81070913 (to ZYH).
P-Reviewers: Gaikwad S, Fernandez M; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Brooks W, de Souza M, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 2.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 3.Certo M, Endo Y, Ohta K, Sakurada S, Bagetta G, Amantea D. Activation of RXR/PPARgamma underlies neuroprotection by bexarotene in ischemic stroke. Pharmacol Res. 2015;102:298–307. doi: 10.1016/j.phrs.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Cheng YC, Anderson CD, Bione S, Keene K, Maguire JM, Nalls M, Rasheed A, Zeginigg M, Attia J, Baker R, Barlera S, Biffi A, Bookman E, Brott TG, Brown RD, Jr, Chen F, Chen WM, Ciusani E, Cole JW, Cortellini L, et al. Are myocardial infarction-associated single-nucleotide polymorphisms associated with ischemic stroke? Stroke. 2012;43:980–986. doi: 10.1161/STROKEAHA.111.632075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci. 2007;28:244–249. doi: 10.1016/j.tips.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Deng S, Zhu G, Liu F, Zhang H, Qin X, Li L, Zhiyi H. CYP4F2 gene V433M polymorphism is associated with ischemic stroke in the male Northern Chinese Han population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:664–668. doi: 10.1016/j.pnpbp.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Evans DM, Marchini J, Morris AP, Cardon LR. Two-stage two-locus models in genome-wide association. PLoS Genet. 2006;2:e157. doi: 10.1371/journal.pgen.0020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Feigin VL, Wang Y, Wang W. The burden of stroke in China: Results from a nationwide population-based epidemiological survey. PLoS One. 2018;13:e0208398. doi: 10.1371/journal.pone.0208398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher RA. On the correlation between relatives on the supposition of Mendelian inheritance. Trans Med Chir Soc Edinb. 1918;52:399–433. [Google Scholar]
- 10.Ivanova EA, Parolari A, Myasoedova V, Melnichenko AA, Bobryshev YV, Orekhov AN. Peroxisome proliferator-activated receptor (PPAR) gamma in cardiovascular disorders and cardiovascular surgery. J Cardiol. 2015;66:271–278. doi: 10.1016/j.jjcc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Janani C, Ranjitha Kumari BD. PPAR gamma gene--a review. Diabetes Metab Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Jih J, Mukherjea A, Vittinghoff E, Nguyen TT, Tsoh JY, Fukuoka Y, Bender MS, Tseng W, Kanaya AM. Using appropriate body mass index cut points for overweight and obesity among Asian Americans. Prev Med. 2014;65:1–6. doi: 10.1016/j.ypmed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasim NB, Huri HZ, Vethakkan SR, Ibrahim L, Abdullah BM. Genetic polymorphisms associated with overweight and obesity in uncontrolled Type 2 diabetes mellitus. Biomark Med. 2016;10:403–415. doi: 10.2217/bmm-2015-0037. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Kim M, Yoo HJ, Shon J, Lee JH. Associations between hypertension and the peroxisome proliferator-activated receptor-delta (PPARD) gene rs7770619 C>T polymorphism in a Korean population. Hum Genomics. 2018;12:28. doi: 10.1186/s40246-018-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BC, Lee HJ, Chung JH. Peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism is associated with reduced risk for ischemic stroke with type 2 diabetes. Neurosci Lett. 2006;410:141–145. doi: 10.1016/j.neulet.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Zhang J, Yu SP. Neuroprotective mechanisms and translational potential of therapeutic hypothermia in the treatment of ischemic stroke. Neural Regen Res. 2017;12:341–350. doi: 10.4103/1673-5374.202915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Cha YS, Park Y, Lee M. PPARgamma2 C1431T polymorphism interacts with the antiobesogenic effects of kochujang, a korean fermented, soybean-based red pepper paste, in overweight/obese subjects: a 12-week, double-blind randomized clinical trial. J Med Food. 2017;20:610–617. doi: 10.1089/jmf.2016.3911. [DOI] [PubMed] [Google Scholar]
- 18.Leng RX, Pan HF, Liu J, Yang XK, Zhang C, Tao SS, Wang DG, Li XM, Li XP, Yang W, Ye DQ. Evidence for genetic association of TBX21 and IFNG with systemic lupus erythematosus in a Chinese Han population. Sci Rep. 2016;6:22081. doi: 10.1038/srep22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Tian Z, Wang M, Kou J, Wang C, Rong X, Li J, Xie X, Pang X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARgamma/Nrf2/NF-kappaB signaling pathway. Int Immunopharmacol. 2019;66:309–316. doi: 10.1016/j.intimp.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, He L, Shi Y. A partition- ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis ( http://analysis.bio-x.cn) Cell Res. 2009;19:519–523. doi: 10.1038/cr.2009.33. [DOI] [PubMed] [Google Scholar]
- 21.Liska MG, Crowley MG, Tuazon JP, Borlongan CV. Neuroprotective and neuroregenerative potential of pharmacologically-induced hypothermia with D-alanine D-leucine enkephalin in brain injury. Neural Regen Res. 2018;13:2029–2037. doi: 10.4103/1673-5374.241427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, He Z, Deng S, Zhang H, Li N, Xu J. Association of adiponectin gene polymorphisms with the risk of ischemic stroke in a Chinese Han population. Mol Biol Rep. 2011;38:1983–1988. doi: 10.1007/s11033-010-0320-y. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Wang LN. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2015:Cd010693. doi: 10.1002/14651858.CD010693.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Bachstetter AD, Cass WA, Lifshitz J, Bing G. Pioglitazone attenuates neuroinflammation and promotes dopaminergic neuronal survival in the nigrostriatal system of rats after diffuse brain injury. J Neurotrauma. 2017;34:414–422. doi: 10.1089/neu.2015.4361. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Wan W, Fang F, Guo L, Zhao Y, Zhang X, Huang F. Clinical relevance of peroxisome proliferator-activated receptor-gamma gene polymorphisms with sepsis. J Clin Lab Anal. 2018;32:e22340. doi: 10.1002/jcla.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu ZM, Hu M, Chan P, Tomlinson B. Early investigational drugs targeting PPAR-alpha for the treatment of metabolic disease. Expert Opin Investig Drugs. 2015;24:611–621. doi: 10.1517/13543784.2015.1006359. [DOI] [PubMed] [Google Scholar]
- 27.Lv X, Zhang L, Sun J, Cai Z, Gu Q, Zhang R, Shan A. Interaction between peroxisome proliferator-activated receptor gamma polymorphism and obesity on type 2 diabetes in a Chinese Han population. Diabetol Metab Syndr. 2017;9:7. doi: 10.1186/s13098-017-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher B. Personal genomes: the case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 29.Marchini J, Donnelly P, Cardon LR. Genome-wide strategies for detecting multiple loci that influence complex diseases. Nat Genet. 2005;37:413–417. doi: 10.1038/ng1537. [DOI] [PubMed] [Google Scholar]
- 30.Meirhaeghe A, Cottel D, Amouyel P, Dallongeville J. Association between peroxisome proliferator-activated receptor gamma haplotypes and the metabolic syndrome in French men and women. Diabetes. 2005;54:3043–3048. doi: 10.2337/diabetes.54.10.3043. [DOI] [PubMed] [Google Scholar]
- 31.Meirhaeghe A, Fajas L, Helbecque N, Cottel D, Auwerx J, Deeb SS, Amouyel P. Impact of the peroxisome proliferator activated receptor gamma2 Pro12Ala polymorphism on adiposity, lipids and non-insulin-dependent diabetes mellitus. Int J Obes Relat Metab Disord. 2000;24:195–199. doi: 10.1038/sj.ijo.0801112. [DOI] [PubMed] [Google Scholar]
- 32.Moffett SP, Feingold E, Barmada MM, Damcott CM, Marshall JA, Hamman RF, Ferrell RE. The C161-->T polymorphism in peroxisome proliferator-activated receptor gamma, but not P12A, is associated with insulin resistance in Hispanic and non-Hispanic white women: evidence for another functional variant in peroxisome proliferator-activated receptor gamma. Metabolism. 2005;54:1552–1556. doi: 10.1016/j.metabol.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Peng B, Ni J, Anderson CS, Zhu Y, Wang Y, Pu C, Wu J, Wang J, Zhou L, Yao M, He J, Shan G, Gao S, Xu W, Cui L Investigators S. Implementation of a structured guideline-based program for the secondary prevention of ischemic stroke in China. Stroke. 2014;45:515–519. doi: 10.1161/STROKEAHA.113.001424. [DOI] [PubMed] [Google Scholar]
- 34.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR American Heart Association Stroke C. 2015 AHA/ASA focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015 doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 35.Prabhu S, Pe’er I. Ultrafast genome-wide scan for SNP-SNP interactions in common complex disease. Genome Res. 2012;22:2230–2240. doi: 10.1101/gr.137885.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priya SS, Sankaran R, Ramalingam S, Sairam T, Somasundaram LS. Genotype phenotype correlation of genetic polymorphism of PPAR gamma gene and therapeutic response to pioglitazone in type 2 diabetes mellitus- a pilot study. J Clin Diagn Res. 2016;10:Fc11–14. doi: 10.7860/JCDR/2016/16494.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranta A, Lichtman JH. Health economics of cerebrovascular disease: can we do better? Neurology. 2015;84:2204–2205. doi: 10.1212/WNL.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 38.Salinas J, Sprinkhuizen SM, Ackerson T, Bernhardt J, Davie C, George MG, Gething S, Kelly AG, Lindsay P, Liu L, Martins SC, Morgan L, Norrving B, Ribbers GM, Silver FL, Smith EE, Williams LS, Schwamm LH. An international standard set of patient-centered outcome measures after stroke. Stroke. 2016;47:180–186. doi: 10.1161/STROKEAHA.115.010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 40.Stefan N, Fritsche A, Haring H, Stumvoll M. Effect of experimental elevation of free fatty acids on insulin secretion and insulin sensitivity in healthy carriers of the Pro12Ala polymorphism of the peroxisome proliferator--activated receptor-gamma2 gene. Diabetes. 2001;50:1143–1148. doi: 10.2337/diabetes.50.5.1143. [DOI] [PubMed] [Google Scholar]
- 41.Stumvoll M, Wahl HG, Loblein K, Becker R, Machicao F, Jacob S, Haring H. Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma2 gene is associated with increased antilipolytic insulin sensitivity. Diabetes. 2001;50:876–881. doi: 10.2337/diabetes.50.4.876. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Huang D, Wang H, Zhou B, Wu X, Ma B, Shi J. Association between serum copeptin and stroke in rural areas of northern china: a matched case-control study. Dis Markers. 2018;2018:9316162. doi: 10.1155/2018/9316162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volcik KA, Nettleton JA, Ballantyne CM, Boerwinkle E. Peroxisome proliferator-activated receptor [alpha] genetic variation interacts with n-6 and long-chain n-3 fatty acid intake to affect total cholesterol and LDL-cholesterol concentrations in the atherosclerosis risk in communities study. Am J Clin Nutr. 2008;87:1926–1931. doi: 10.1093/ajcn/87.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan J, Xiong S, Chao S, Xiao J, Ma Y, Wang J, Roy S. PPARgamma gene C161T substitution alters lipid profile in Chinese patients with coronary artery disease and type 2 diabetes mellitus. Cardiovasc Diabetol. 2010;9:13. doi: 10.1186/1475-2840-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Wang Q, Yin Y, Yang Z, Li W, Liang D, Zhou P. Association between peroxisome proliferator-activated receptor gamma gene polymorphisms and atherosclerotic diseases: a meta-analysis of case-control studies. J Atheroscler Thromb. 2015;22:912–925. doi: 10.5551/jat.26138. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Xu L, Jonas JB, You QS, Wang YX, Yang H. Prevalence and associated factors of dyslipidemia in the adult Chinese population. PloS one. 2011;6:e17326. doi: 10.1371/journal.pone.0017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL Investigators NE-C. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017a;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 48.Wang XL, Oosterhof J, Duarte N. Peroxisome proliferator-activated receptor gamma C161-->T polymorphism and coronary artery disease. Cardiovasc Res. 1999;44:588–594. doi: 10.1016/s0008-6363(99)00256-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, He Z, Deng S. Ursolic acid reduces the metalloprotease/anti-metalloprotease imbalance in cerebral ischemia and reperfusion injury. Drug Des Devel Ther. 2016a;10:1663–1674. doi: 10.2147/DDDT.S103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Yin X, Li L, Deng S, He Z. Association of apolipoprotein c3 genetic polymorphisms with the risk of ischemic stroke in the northern Chinese han population. PLoS One. 2016b;11:e0163910. doi: 10.1371/journal.pone.0163910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Liu F, Li L, Deng S, He Z. The association between apolipoprotein A1-C3-A5 gene cluster promoter polymorphisms and risk of ischemic stroke in the northern Chinese Han population. J Int Med Res. 2017b;45:2042–2052. doi: 10.1177/0300060517713517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei WM, Wu XY, Li ST, Shen Q. PPARG gene C161T CT/TT associated with lower blood lipid levels and ischemic stroke from large-artery atherosclerosis in a Han population in Guangdong. Neurol Res. 2016;38:620–624. doi: 10.1080/01616412.2016.1189056. [DOI] [PubMed] [Google Scholar]
- 53.Wippold FJ, 2nd Expert Panel on Neurologic I. Focal neurologic deficit. AJNR Am J Neuroradiol. 2008;29:1998–2000. [PMC free article] [PubMed] [Google Scholar]
- 54.Wu XJ, Sun XH, Wang SW, Chen JL, Bi YH, Jiang DX. Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPAR gamma. Eur Rev Med Pharmacol Sci. 2018;22:5688–5696. doi: 10.26355/eurrev_201809_15836. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z, Lou Y, Jin W, Liu Y, Lu L, Lu G. The C161T polymorphism in the peroxisome proliferator-activated receptor gamma gene (PPARgamma) is associated with risk of coronary artery disease: a meta-analysis. Mol Biol Rep. 2013;40:3101–3112. doi: 10.1007/s11033-012-2384-3. [DOI] [PubMed] [Google Scholar]
- 56.Xia P, Pan Y, Zhang F, Wang N, Wang E, Guo Q, Ye Z. Pioglitazone confers neuroprotection against ischemia-induced pyroptosis due to its inhibitory effects on HMGB-1/RAGE and Rac1/ROS pathway by activating PPAR. Cell Physiol Biochem. 2018;45:2351–2368. doi: 10.1159/000488183. [DOI] [PubMed] [Google Scholar]
- 57.Xie HJ, Hai B, Wu M, Chen Q, Liu MM, Dong C, Guo ZR. Analysis on the association between PPARalpha/gamma polymorphisms and lipoprotein(a) in a Chinese Han population. Mol Genet Genomics. 2014;289:981–987. doi: 10.1007/s00438-014-0866-9. [DOI] [PubMed] [Google Scholar]
- 58.Ye E, Yang H, Chen L, Chen Q, Sun M, Lin Z, Yu L, Peng M, Zhang C, Lu X. Adiponectin and peroxisome proliferator-activated receptor-gamma gene polymorphisms and gene-gene interactions with type 2 diabetes. Life Sci. 2014;98:55–59. doi: 10.1016/j.lfs.2013.12.232. [DOI] [PubMed] [Google Scholar]
- 59.Yilmaz-Aydogan H, Kurnaz O, Kurt O, Akadam-Teker B, Kucukhuseyin O, Tekeli A, Isbir T. Effects of the PPARG P12A and C161T gene variants on serum lipids in coronary heart disease patients with and without Type 2 diabetes. Mol Cell Biochem. 2011;358:355–363. doi: 10.1007/s11010-011-0987-y. [DOI] [PubMed] [Google Scholar]
- 60.Youssef SM, Mohamed N, Afef S, Khaldoun BH, Fadoua N, Fadhel NM, Naceur SM. Combined effects of the C161T and Pro12Ala PPARgamma2 gene variants with insulin resistance on metabolic syndrome: a case-control study of a central Tunisian population. J Mol Neurosci. 2014;52:487–492. doi: 10.1007/s12031-013-0161-y. [DOI] [PubMed] [Google Scholar]
- 61.Yuan G, Chen X, Li D. Modulation of peroxisome proliferator-activated receptor gamma (PPAR gamma) by conjugated fatty acid in obesity and inflammatory bowel disease. J Agric Food Chem. 2015;63:1883–1895. doi: 10.1021/jf505050c. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Song C, Li H, Hou J, Li D. Ursolic acid prevents augmented peripheral inflammation and inflammatory hyperalgesia in high-fat diet-induced obese rats by restoring downregulated spinal PPARalpha. Mol Med Rep. 2016;13:5309–5316. doi: 10.3892/mmr.2016.5172. [DOI] [PubMed] [Google Scholar]
- 63.Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci. 2009;29:6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.