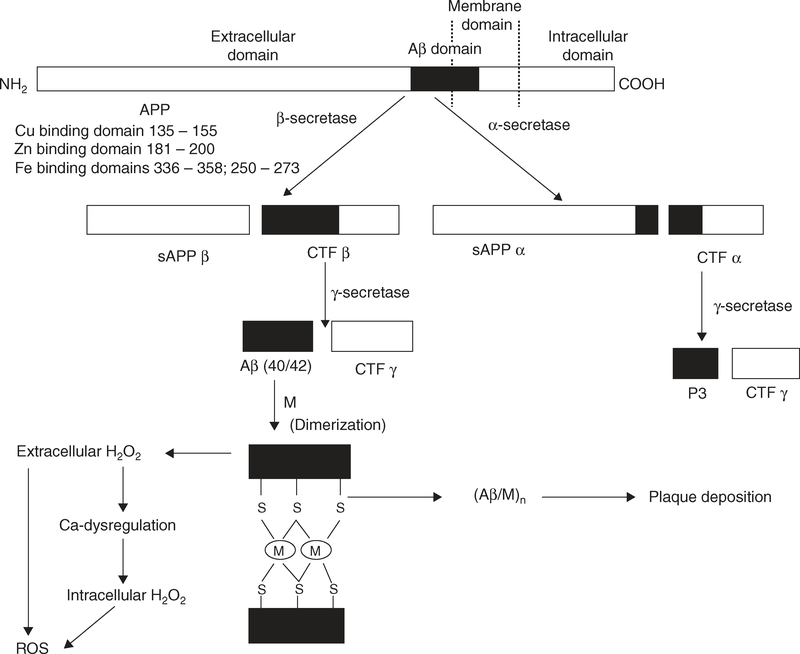
Figure 2. The proteolytic processing of amyloid precursor protein (APP) to produce Aβ that coordinates the metal ions (M: Zn, Cu and Fe) to induce aggregation, and generation of ROS.
APP (695, 751, 770 amino acid isoforms that predominate in brain) can be processed in the plasma membrane as it travels from the intracellular origin to extracellular matrix through the non-amyloidogenic route that involves cleavage at the α-secretase site at amino acid 17 of the 40 – 42 amino acid Aβ domain resulting in two fragments, sAPPα and a C-terminal fragment (CTFα). Further proteolysis of the CTFα fragment by α-secretase generates the non-amyloidogenic peptide p3 and a C-terminal fragment CTFγ. When APP escapes processing at the α-site, it undergoes β-secretase cleavage at the beginning of Aβ domain, resulting in a C-terminal fragment CTFβ and sAPPβ. Next, the resultant β-stub becomes the substrate for γ-secretase cleavage, culminating in extracellular Aβ secretion. The hyper-metallated (by Zn, Fe and Cu) state of Aβ as a consequence of age-dependent elevations in tissue metal concentrations can induce Aβ aggregation. H2O2 can initiate a number of oxidative events, including Fenton reactions to form toxic hydroxyl radicals and calcium dysregulation, and subsequent reactive oxygen species (ROS) generation.