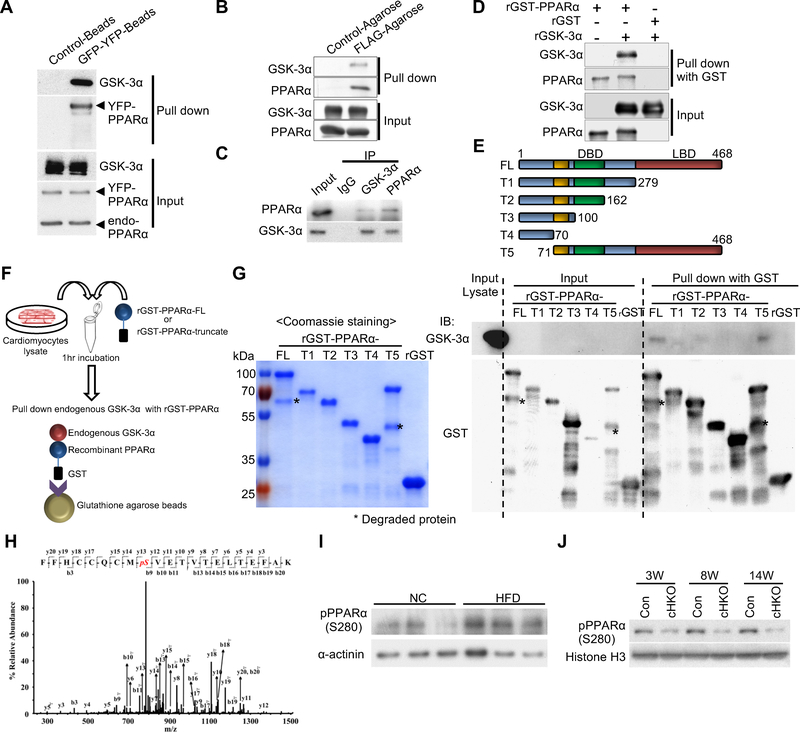
Figure 2. GSK-3α physically interacts with and phosphorylates PPARα at Ser280 in cardiomyocytes (CMs) and in the heart.
(A and B) Immunoprecipitation assays to test the interaction between endogenous GSK-3α and exogenously expressed PPARα. YFP-tagged PPARα or FLAG-tagged PPARα was overexpressed in CMs using adenovirus (A) or in transgenic mouse hearts under the control of the αMHC-promoter (B), respectively. (C) Co-immunoprecipitation assays testing the interaction between endogenous GSK-3α and endogenous PPARα in CMs. (D) In vitro binding assays testing the direct interaction between recombinant (r) GSK-3α and rPPARα. (E to G) Immunoprecipitation assays to identify the amino acids in PPARα responsible for the interaction with endogenous GSK-3α. (E) Schematic representation of rGST-fused PPARα fragments. (F) Schema of the immunoprecipitation assays. rGST-fused-PPARα-full length (FL) or truncated PPARα (T1 to 5) was incubated with lysates extracted from cultured CMs, followed by pull-down with glutathione-sepharose and immunoblotting with anti-GSK-3α antibody. (G) Coomassie Brilliant Blue staining of rGST-PPARα-FL or truncated rGST-PPARα (T1 to T5) (left). Immunoblots testing the binding of endogenous GSK-3α to rGST-PPARα-FL or T1 to T5 (right). (H) Mass spectrometry analysis of the rGST-PPARα protein phosphorylated by GSK-3α in a kinase reaction. The MS/MS spectrum of the PPARα residue corresponding to Ser280 was increased at 80 Da, indicating phosphorylation. (I) Immunoblots showing Ser280 phosphorylation of endogenous PPARα in the hearts of WT mice fed a high-fat diet (HFD) or normal chow (NC) for 3 weeks. α-sarcomeric actinin was used as a loading control. (J) Immunoblots showing pPPARα (S280) in the hearts of control or GSK-3α cHKO mice fed a HFD for the indicated period. See also Figure S3.