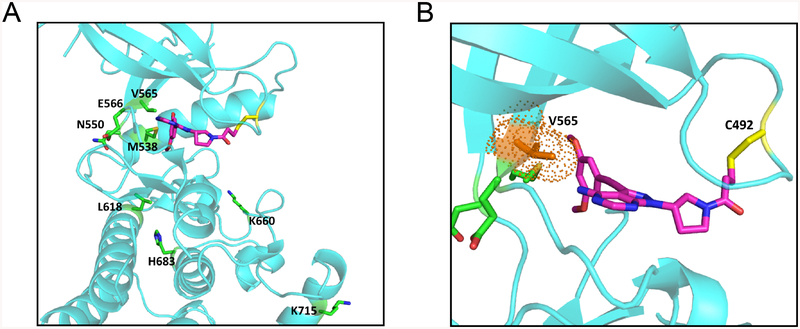
Figure 4. Structural modeling of secondary FGFR2 kinase domain mutations with TAS-120.
A, Model showing TAS-120 docked into ATP-binding pocket of wild type FGFR2. Amino acid residues corresponding to mutations conferring resistance to ATP competitive FGFR inhibitors are highlighted. Structural representations were prepared using PyMOL.
B, A close-up view of TAS-120 in ATP-binding pocket of wild type FGFR2. The gatekeeper residue (V565) is in close proximity to dimethoxy phenyl group of TAS-120.