Abstract
Background
Children and young people (CYP) with chronic rheumatic conditions; Juvenile Idiopathic Arthritis, Juvenile Systemic Lupus Erythematosus, Juvenile Dermatomyositis and Juvenile Vasculitis, treated with steroids, have low bone density, increased fracture risk and are likely to have suboptimal peak bone mass. There is currently no evidence base for the management of steroid-induced bone loss in children with rheumatic diseases.
Methods
We undertook a multi-centre double dummy double-blind randomised placebo controlled trial to investigate whether the bisphosphonate risedronate was superior to alfacalcidol or calcium and vitamin D supplementation in the prevention and treatment of steroid-induced osteopaenia in these children. Patients were stratified and randomised in a 1:1 ratio, into: placebo; alfacalcidol; risedronate. The primary outcome was the change in lumbar spine bone mineral density z score (LSaBMDz) measured by dual energy x-ray absorptiometry at one year. Secondary outcome was fracture rate.
Results
Two hundred and seventeen patients were recruited to the study. Seventy seven placebo, 71 alfacalcidol, and 69 risedronate. Highly statistically significant differences were observed in the change in LSaBMDz between the placebo and risedronate groups; 0.274, 95% CI (0.061, 0.487) (p < 0.001) and between the risedronate and the alfacalcidol groups; 0.326 95% CI (0.109, 0.543) (p < 0.001). The difference observed between the alfacalcidol and placebo group was not statistically significant.
Highly statistically significant differences were seen in the change in Total Body Less Head aBMD-Z Score between the placebo and risedronate groups (p < 0.01) but not between the alfacalcidol and risedronate groups. No significant differences in fracture frequency, adverse or serious adverse reactions were observed between the groups.
Conclusions
Children and adolescents receiving steroids for rheumatic diseases benefit from prophylactic treatment with bisphosphonates to increase LSaBMD. Alfacalcidol is ineffective.
Keywords: Juvenile idiopathic arthritis, Steroids, Osteoporosis, Bisphosphonates, Clinical trial
Highlights
-
•
First randomised controlled trial into the prevention and treatment of steroid-induced osteopenia in children.
-
•
Risedronate significantly increases BMD z score compared to Vit D analogues and placebo Vit D analogues do not increase BMDz scores over placebo.
-
•
The bisphosphonate Risedronate was well tolerated and no significant side effects were observed.
Research in context
Many children and young people with rheumatic diseases are treated with steroids for long periods. This, despite the advent of biological therapies. Osteoporosis is one of the major complications of steroids. For adults there are currently numerous evidence – based therapeutic strategies to prevent bone loss. No such evidence exists for children and young people.
When the British Society for Paediatric Rheumatology (BSPAR) surveyed paediatric rheumatologists in the UK regarding their practice, in their children and young people treated with steroids, there was no consensus on which drugs to use for skeletal protection (personal communication).
Calcium and vitamin D supplementation, or bisphosphonates in those at higher risk, form the basis of current preventative treatment strategies.
Older studies suggested calcitriol, or the calcitriol precursor alfacalcidol, increased areal BMD in adults with steroid-induced osteoporosis.
Two trials have studied the efficacy of bisphosphonates in children with rheumatic diseases; one open label study demonstrated an improvement in aBMD whilst an underpowered RCT showed no improvement. Two randomised trials, using bisphosphonates, have been registered but both were abandoned due to failure to recruit. This demonstrates the difficulty of undertaking such trials in children and young people who are often quite ill due to their disease and parents reluctance to engage in additional drug therapies. A recent metanalysis found that the largest published prospective treatment study included only 44 children. A 2007 Cochrane review concluded that there was insufficient evidence to recommend bisphosphonates as standard therapy for the treatment of secondary paediatric osteoporosis.
This is therefore the first and only fully powered randomised controlled trial to investigate whether the bisphosphonate risedronate or alfacalcidol results in clinical meaningful reduction in bone loss in children and young people with rheumatic diseases treated with steroids.
The primary aim of this study was to determine the efficacy of risedronate or alfacalcidol compared to placebo in increasing lumbar spine aBMD z-score in children with rheumatic diseases, with the secondary aim of assessing the effect of the interventions on fracture rate.
The results of this trial will be of great value to paediatricians and clinicians treating children and young people with steroids providing the only evidence –base for the efficacy of a bisphosphonate in this situation.
Alt-text: Unlabelled Box
1. Background
Chronic rheumatic diseases of childhood affect between 1 and 3 per 1000 children in the UK [1], [2]. Bone loss is a well-recognized major complication with considerable morbidity [3]. Major contributory factors include: the underlying inflammatory process [4] altered nutrition [5], growth impairment [6], reduced physical activity [7] and treatment, particularly glucocorticoids [3], [8], [9]. Steroids are detrimental to bone stock, however, despite the introduction of biologic drugs, for many children steroids remain the only means by which their disease can be controlled. Tens of thousands of children world-wide currently receive steroids for chronic rheumatic conditions. Steroids reduce peak bone mass and fracture risk increases as steroid dose increases [3], [9], [10]. Many more children receive recurrent courses of steroids for other common diseases such as asthma, with an associated increased risk of fracturing [11], [12].
Children receiving steroids can, unlike adults, dramatically increase their BMD when their disease is brought under control [13]. There is thus a tension between effective disease control and the adverse effects of the steroid therapy.
The current guidelines for adults are that those treated with GCs should receive prophylactic treatment for the prevention of bone loss [14]. No such recommendations are in place for children.
When the British Society for Paediatric Rheumatology (BSPAR) surveyed paediatric rheumatologists in the UK regarding their practice, there was no consensus on which drugs to use for skeletal protection (personal communication).
Calcium and vitamin D supplementation, or bisphosphonates in those at higher risk, form the basis of current preventative treatment strategies.
Older studies suggested calcitriol, or the calcitriol precursor alfacalcidol, increased aBMD in adults with steroid-induced osteoporosis [15], [16].
Two trials have studied the efficacy of bisphosphonates in children with rheumatic diseases; one open label study demonstrated an improvement in aBMD whilst an underpowered RCT showed no improvement [17], [18]. A 2007 Cochrane review concluded that there was insufficient evidence to recommend bisphosphonates as standard therapy for the treatment of secondary paediatric osteoporosis [19]. A recent metanalysis found that the largest published prospective treatment study included only 44 children [20].
The primary aim of this study was to determine the efficacy of risedronate or alfacalcidol compared to placebo in increasing lumbar spine aBMD z-score in children with rheumatic diseases, with the secondary aim of assessing the effect of the interventions on fracture rate.
2. Methods
This randomised double blind placebo controlled trial was conducted on behalf of the British Society of Paediatric and Adolescent Rheumatology (BSPAR); the study was approved by OREC Northern Ireland and registered with EuDRACT No: 2005-003129-23; ISRCTN66814619.
Patients were recruited from eleven sites throughout the UK. Trial duration was one year with three months post-trial follow-up. The trial ran from 22/Aug/2007 to 27/Feb/2013.
Children and adolescents with Juvenile Idiopathic Arthritis (JIA), Juvenile Systemic Lupus Erythematosus (JSLE), Juvenile Dermatomyositis (JDM) or vasculitis, between the ages of four and 18 years, commencing or established on steroid therapy, were eligible to participate. Protocol details are in Supplementary data.
Written informed consent was obtained from all parents/guardians and consent/assent obtained from all patients prior to starting the study.
Participants were stratified according to:
-
⁎
Tanner stage (0–2; 3–5)
-
⁎
Steroid dose (prednisolone equivalent) (Low ≤ 0.2 mg/kg/d) Vs medium-high (> 0.2 mg/kg/d)
Subjects were randomised in an independent central randomisation facility (Clinical Research Support Centre NI) into three groups, according to stratification information included in the registration form, in a1:1 ratio to receive:
Group 1. Alfacalcidol/risedronate placebo.
Group 2. Alfacalcidol 15 ng/kg/day (max 1 μg).
Group 3. Risedronate 1 mg/kg/week for body weight < 30kgs, or 35 mg/wk. for body weight > 30 kgs.
The risedronate and risedronate placebo were identical as were the alfacalcidol and placebo.
All children received a supplement of 500 mg calcium and 400 IU vitamin D daily.
The research nurse faxed the registration information to the CRSC where they were randomised to receive a study number which the research nurse obtained by Fax. Simultaneously the trial pharmacist in each study centre received the same number to dispense the appropriated coded and blinded medication. The study pharmacists were not blinded to the treatment dispensed. They had no further involvement in the study. All persons involved in the study: staff, patients and parents were blinded to the study treatment.
2.1. Visit schedule
Patients were assessed and randomised to treatment group at T0, and assessed every three months until study end. Throughout the trial, changes in anti-rheumatic therapy, including steroid dosage, were permitted according to locally-defined clinical need.
2.2. Clinical assessment
The clinical parameters assessed at each visit were; height, weight skin fold thickness, Tanner score, JIA disease activity, (Childhood Health Assessment Questionnaire, British Isles Lupus Assessment Group Index, Childhood Myositis Assessment Score, fracture history. All menstruating females were tested for pregnancy.
2.3. Laboratory measurements
The following parameters were assessed in blood and urine at each visit. Samples were not obtained fasting as this was logistically difficult in children and would not be feasible in normal clinical practice.
Full blood picture (FBP); Erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); Urea and Electrolytes (U&E); Bone profile; Urine Calcium/Creatinine ratio every 3 months. All centres used their hospital's standard ranges which were stored in the site files. Serum 25 hydroxyvitamin D (Immunodiagnostics, UK) and parathormone (Abbot Diagnostics, UK), bone alkaline phosphatase, Osteocalcin (measures of bone formation), and Crosslaps (measure of bone resorption) (Roche diagnostics, UK) were measured at T = 0 and at 3 months. The latter three were analysed centrally (Belfast Hospital Trust). Paediatric reference range was used for Crosslaps [21].
2.4. Radiological assessment
In the 11 participating centres, seven DXA scanners were Lunar Prodigy and four were Hologic. All participating DXA scanners were standardised using a paediatric spine phantom (MRC Mineral Metabolism Unit, Leeds, UK) [22]. The initial DXA scans were performed within two weeks of commencing the study and lumbar spine areal BMD (LSaBMD) and total body less head areal BMD (TBLHaBMD) were measured then and at visit 3 (6 months) and at visit 5 (12 months). LSaBMD and TBLHaBMD z-scores were either machine-derived or, for five patients under age five years, calculated by the Least Median Squares (LMS) method using UK reference data [23], [24].
Lateral radiographs of the spine for vertebral deformity were performed at baseline and month 12. All radiographs were read centrally and blindly by a consultant musculoskeletal radiologist (ME). Vertebral fractures were scored 0–3 using the Genant method [25] where grade 0: normal; grade 1: mild fracture, 20% to 25% loss of height; grade 2: moderate fracture, 25% to 40% loss of height; grade 3: severe fracture, greater than 40% loss of height.
2.5. Safety
Urinary calcium excretion was recorded as a safety measure. The numbers of patients experiencing adverse events (AEs) or serious adverse events (SAEs) and number of events are reported by treatment group. An adverse event is any untoward medical occurrence in a patient in the study. An adverse reaction (AR) is defined as any AE considered to have a possible, probable or definite relationship to the study drug.
2.6. Study details
Risedronate and its placebo were provided by Proctor and Gamble (subsequently Warner Chilcott UK). Alfacalcidol was obtained from Leo Laboratories (One Alpha, Leo laboratories) and the alfacalcidol placebo was made by Victoria Pharmaceuticals, Belfast Hospital Trust. Calcium and vitamin D supplements were supplied by Victoria Pharmaceuticals.
None of the pharmaceutical companies had any input into the trial design or data analysis.
2.7. Study oversight
The study was designed by the authors and the trial data input and analysis undertaken by the Northern Ireland Clinical Trials Unit (NICTU). The progress of the trial was monitored by the Data Monitoring and Ethics Committee of Arthritis Research UK (now Versus Arthritis). Data analysis was conducted by three of the authors.
2.8. Sample size calculation
There was little existing evidence on what was regarded as a clinically meaningful effect size.
The initial required sample size was 270 children. To detect an improvement between the treatment groups of 6.25 and between the treatment groups and the control groups of 6.25, using a SD of 12.5, observed in our 1 year growth hormone study [6], 75 children were required in each of the three study arms; with 80% power to detect a significant difference at the 5% level of significance. We further expected a dropout rate of 15%, and that approximately 20% of this population would not receive steroids for one year. Thus to ensure that an adequate number of children would complete the study on steroids we required 90 children per treatment group; a total of 270.
Interim analysis showed that there was much better retention rate and fewer stopping steroids. There were two re-assessments of sample size calculation. One assessment after reducing percentage of patients without efficacy endpoint as 15% and another re-calculation was done with 10% dropouts. The effect size was kept the same. The sample size was set to 216, with 10% drop out rate.
2.9. Statistical analysis
The efficacy measure was the change in BMD at year 1 follow-up from baseline. The primary outcome measure was the change from baseline in LSaBMD z-score and the primary analysis was carried out using ANOVA, to test whether there is a statistically significant difference between the groups. The post-hoc bonferroni test was carried out to check which pair differ significantly. (Placebo vs. Alfacalcidol, Placebo vs. Risedronate, Alfacalcidol vs. Risedronate).
Analysis of covariance was performed on Lumbar spine BMD at year 1 adjusting for Lumbar spine BMD at baseline, age and gender. A post-hoc pairwise comparison was carried out using Bonferroni test.
Patients whose drug was prematurely withdrawn were encouraged to have a DXA scan at one year and their results included in the analysis.
Intention-to- treat (ITT) population includes all patients who were randomised and who received at least one dose of the planned study medication. Per protocol analysis includes all the patients who received all doses of the study drug as per protocol.
The primary efficacy analysis was based on the ITT population, but a per-protocol analysis was also performed. Final analysis was unadjusted for the interim analysis.
The Secondary Outcome was the rate of vertebral or other fractures during the trial period.
Count of observations, mean ± SD, median (p25 to p50), and frequency (percentage) were used to summarise the variables.
3. Results
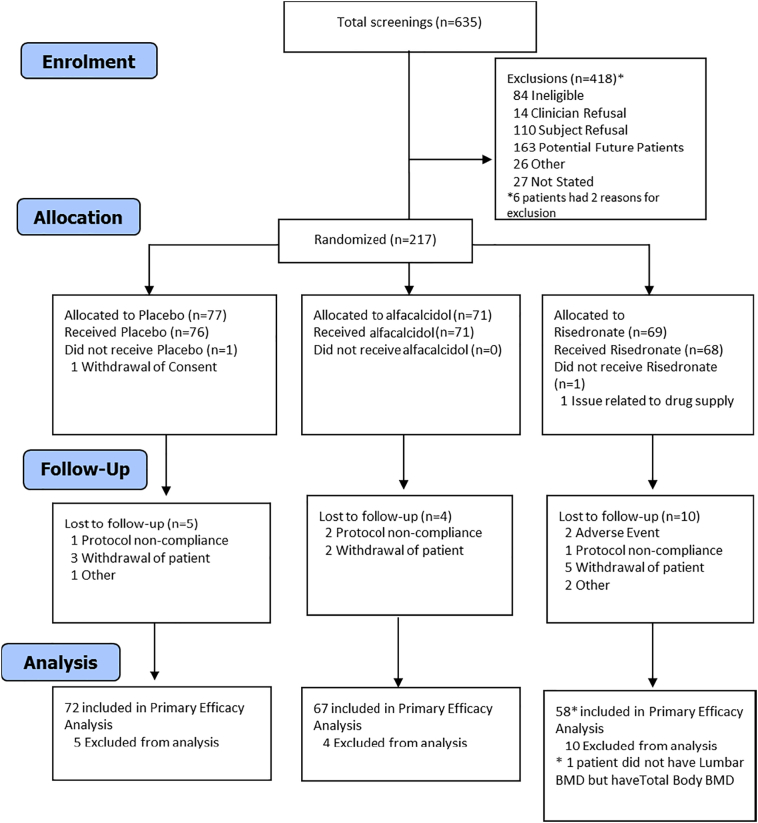
Two hundred and seventeen patients were recruited from 11 participating centres throughout the UK and randomised to placebo (n = 77), alfacalcidol (n = 71), or risedronate (n = 69). Screening and recruitment information are shown in the consort flow diagram Fig. 1.
Fig. 1.
CONSORT flow diagram.
Baseline demographics are shown in Table 1.
Table 1.
Baseline characteristics (population: intention to treat (ITT).
| Variables |
Placebo |
Alfacalcidol |
Risedronate |
All Patients |
|
|---|---|---|---|---|---|
| N | 77 | 71 | 69 | 217 | |
| Age (years), mean (SD) | 12.1 (3.5) | 12.1 (3.7) | 12.0 (3.4) | 12.1 (3.5) | |
| Gender | Female, n (%) | 55 (71.4) | 48 (67.6) | 53 (76.8) | 156 (71.9) |
| Male, n (%) | 22 (28.6) | 23 (32.4) | 16 (23.2) | 61 (28.1) | |
| Tanner score, median (IQR) | 2 (1 to 4) | 2 (1 to 4) | 2 (1 to 3) | 2 (1 to 4) | |
| Steroid dose, n (%) | ≤ 0.2 mg/kg | 37 (48.0) | 30 (42.2) | 32 (46.4) | 99 (45.6) |
| > 0.2 mg/kg | 40 (52.0) | 41 (57.8) | 37 (53.6) | 118 (54.4) | |
| Ethnic origin, n (%) | Caucasian | 59 (76.6) | 54 (76.1) | 55 (79.7) | 168 (77.4) |
| Black | 4 (5.2) | 4 (5.6) | 6 (8.7) | 14 (6.4) | |
| Oriental | 0 (0) | 1 (1.4) | 0 (0) | 1 (0.5) | |
| Asian | 11 (14.3) | 10 (14.1) | 6 (8.7) | 27 (12.4) | |
| Other | 3 (3.9) | 2 (2.8) | 2 (2.9) | 7 (3.2) | |
| Disease group, n (%) | JIA | 21 (27.3) | 30 (42.2) | 20 (29.0) | 71 (32.7) |
| JSLE | 31 (40.3) | 21 (29.6) | 24 (34.8) | 76 (35.0) | |
| JDM | 17 (22.1) | 13 (18.3) | 16 (23.2) | 46 (21.2) | |
| Vasculitis | 11 (14.3) | 12 (16.9) | 13 (18.8) | 36 (16.6) | |
| Approximate cumulative steroid dose mg, mean (SD) (n = 206)a | 8403.7 (9206.9) | 9108.7 (7528.0) | 8090.4 (9390.1) | 8531.5 (8721.8) | |
| Relevant medical conditionsb (yes), n (%) | 42 (55.3) | 39 (54.9) | 43 (62.3) | 124 (57.4) | |
| On any medications at baseline | 75 (98.7) | 69 (97.2) | 68 (98.6) | 212 (98.2) | |
| DMARDSc Methotrexate, Mycophenolate Mofetil, Azathioprine, Cyclophosphamide, Hdroxychloroquine, Cyclosporine |
71 (93.4) | 64 (90.1) | 62 (89.9) | 197 (91.2) | |
| Biologics: Etanercept; Infliximab; Anakinra; Tocilizumab | 8 (10.5) | 17 (23.9) | 7 (10.1) | 32 (14.8) | |
| Prior fracture history (yes), n (%) | 13 (17.1) | 9 (12.7) | 8 (11.6) | 30 (13.89) | |
One patient in placebo had a cumulative steroid dose of 238,325 mg and one patient in the alfacalcidol arm had a cumulative dose of 487,400 mg. These values were excluded in the mean (SD) calculation.
Details of relevant medical conditions are available in Table 1.
Disease modifying anti rheumatic drugs.
Ethnicity, gender, age, disease subtype, Tanner stage and steroid dose were evenly distributed between the three groups. There was no significant difference in the fracture history between the groups.
Some 90% of participants in each group were concurrently treated with a DMARD and biologic use was 10.5%, 23.9% and 10.1% in the three groups respectively. Five patients in the placebo group started steroids de novo and two in the alfacalcidol group. All other patients were already taking steroids at the start of the study. The calculated cumulative steroid dose from the available patient records, prior to the commencement of the trial averaged 8–9 g in each group. Details of relevant medical conditions are available in Table 1 of Supplementary data.
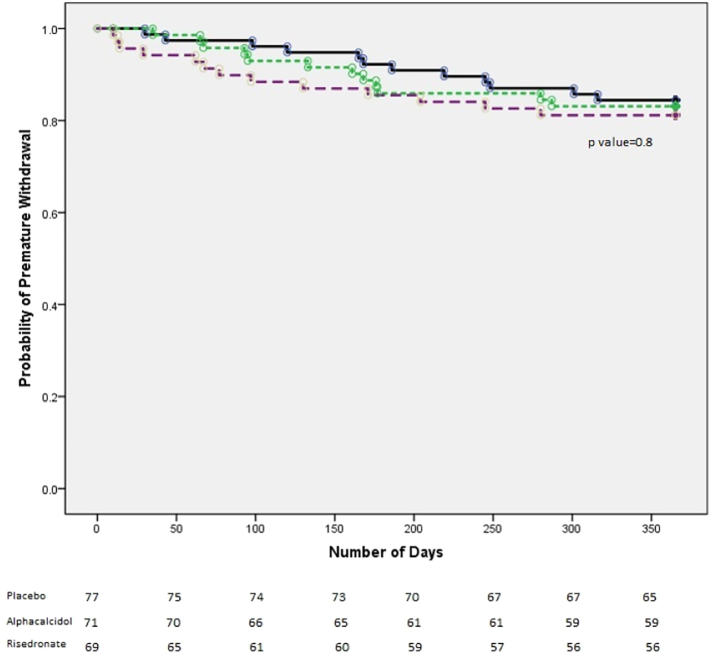
Kaplan Meier survival curves demonstrate similar retention rates between the three groups Fig. 2, although a slightly earlier drop out was noted in the risedronate group, there was no statistical difference between them.
Fig. 2.
Kaplan-Meier survival curves. Time to study drug withdrawal according to treatment group (population: ITT).
Eighty four percent, 86% and 81% of the placebo, alfacalcidol and risedronate groups respectively were still taking steroids at one year with mean daily intake of 5.88; 5.82 and 6.17 mg of prednisolone daily respectively. Clinical indicators of disease activity were similar at one year as were biochemical markers of disease activity Table 2.
Table 2.
Steroid doses anthropometric values and disease activity markers at baseline and one year (population: ITT).
| Variable | Visit | Mean (SD) |
||
|---|---|---|---|---|
| Placebo | Alfacalcidol | Risedronate | ||
| Daily Prednisolonea Mean ± SD |
Baseline | 76 12.20 (8.83) |
70 13.83 (10.97) |
69 16.91 (22.69) |
| One year | 65 5.88 (6.40) |
59 5.82 (4.83) |
55 6.17 (5.44) |
|
| Height cms | Baseline | 145.0 (18.6) | 145.3 (21.3) | 144.6 (19.9) |
| One year | 149.0 (15.5) | 149.9 (20.2) | 148.7 (19.2) | |
| Weight Kg | Baseline | 46.14 (17.41) | 48.24 (20.79) | 48.05 (18.87 |
| One year | 49.82 (17.85) | 53.62 (23.08) | 51.56 (18.79) | |
| Hb g/dl | Baseline | 12.8 (1.36) | 12.6 (1.31) | 12.5 (1.25) |
| One year | 12.6 (1.53) | 12.5 (1.14) | 12.5 (1.12) | |
| ESR mm/h | Baseline | 14.7 (17.1) | 16.2 (13.6) | 14.7 (16.7) |
| One year | 11.0 (11.7) | 15.9 (17.8) | 16.0 (21.5) | |
| CRP mg/L | Baseline | 5.2 (5.8) | 7.8 (11.4) | 7.8 (9.5) |
| One year | 4.8 (5.2) | 6.2 (6.2) | the5.7 (5.7) | |
| ALP IU/L | Baseline | 187.0 (126.6) | 196.1 (133.2) | 179.1 (112.4) |
| One year | 217.8 (158.5) | 233.4 (191.9) | 214.9 (155.8) | |
One in placebo, 4 in alfacalcidol and 4 in risedronate groups. For these, the daily prednisolone equivalent was calculated.
Two patients were treated with deflazacort, and one hydrocortisone. Nine patients received methylprednisolone pulses.
3.1. Efficacy analysis
The primary outcome was the change in LSaBMD z-score at one year from baseline which together with LSaBMD, TBLHaBMD, and TBLHaBMD z-score are summarised in Table 3, with the efficacy analysis in Table 4. For transparency the BMD values without and with the calculated results are shown. For all subsequent aBMD results in subsequent tables, the values shown are the calculated values.
Table 3.
Areal BMD gm/cm2 and areal BMD z-scores over during the course of the study (population: ITT).
| N, mean (SD) |
|||
|---|---|---|---|
| Placebo | Alfacalcidol | Risedronate | |
| Lumbar Spine aBMD g/cm2 | |||
| Screening | 76, 0.76 (0.20) | 71, 0.79 (0.21) | 68, 0.78 (0.19) |
| 6 month | 68, 0.79 (0.18) | 63, 0.80 (0.22) | 59, 0.82 (0.19) |
| One-year | 72, 0.80 (0.19) | 67, 0.83 (0.21) | 58, 0.85 (0.17) |
| Lumbar spine aBMD z score | |||
| Screening | 76, − 1.15 (1.15) | 68, − 0.91 (1.04) | 66, − 1.04 (1.17) |
| Screening-calculateda | 76, − 1.15 (1.15) | 71, − 0.96 (1.04) | 68, − 0.99 (1.19) |
| 6 month | 68, − 1.17 (1.07) | 61, − 0.95 (1.12) | 57, − 0.84 (1.16) |
| 6 month-calculateda | 68, − 1.17 (1.07) | 63, − 1.01 (1.15) | 59, − 0.79 (1.18) |
| One-year | 72, − 1.13 (1.10) | 67, − 0.99 (1.07) | 58, − 0.74 (1.17) |
| One-year-calculateda | 72, − 1.13 (1.10) | 67, − 1.00 (1.07) | 58, − 0.75 (1.15) |
| Total body less head aBMD g/cm2 | |||
| Screening | 75, 0.91 (0.14) | 70, 0.93 (0.16) | 68, 0.91 (0.13) |
| 6 month | 68, 0.93 (0.13) | 62, 0.94 (0.17) | 58, 0.93 (0.13) |
| One-year | 70, 0.92 (0.12) | 65, 0.97 (0.16) | 59, 0.96 (0.13) |
| Total body less head aBMD z score | |||
| Screening | 75, − 0.57 (0.99) | 67, − 0.4 (1.09) | 66, − 0.63 (1.08) |
| Screening-calculateda | 75, − 0.57 (0.99) | 70, − 0.63 (1.64) | 68, − 0.65 (1.08) |
| 6 month | 68, − 0.62 (0.99) | 59, − 0.29 (1.19) | 56, − 0.50 (1.08) |
| 6 month-calculateda | 68, − 0.62 (0.99) | 62, − 0.49 (1.55) | 58, − 0.52 (1.08) |
| One-year | 70, − 0.70 (0.94) | 65, − 0.46 (1.28) | 59, − 0.46 (1.09) |
| One-year-calculateda | 70, − 0.70 (0.94) | 65, − 0.57 (1.47) | 59, − 0.44 (1.08) |
Least Median Squares (LMS) method [22].
Includes z-scores for five patients (all visits) who were below five years of age that were calculated using.
Table 4.
Efficacy analysis: change in areal BMD and areal BMD z-scores from baseline to one year (population: ITT).
| Change from baseline to 1 year | Mean (SD) |
p-Value | Alfacalcidol vs placebo |
Risedronate vs placebo |
Risedronate vs alfacalcidol |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Alfacalcidol | Risedronate | Mean difference (95% CI) |
p-Value | Mean difference (95% CI) |
p-Value | Mean difference (95% CI) |
p-Value | ||
| Primary outcome measure: change in lumbar spine aBMD-Z score | ||||||||||
| Primary analysis - ITT | − 0.036 (0.435) | − 0.088 (0.519) | 0.238 (0.551) | 0.0007 | − 0.052 (− 0.257, 0.153) | 1.0 | 0.274 (0.061, 0.487) | 0.007 | 0.326 (0.109, 0.543) | 0.001 |
| Per protocol | − 0.043 (0.431) | − 0.036 (0.510) | 0.264 (0.540) | 0.0008 | 0.007 (− 0.204, 0.219) | 1.0 | 0.307 (0.092, 0.521) | 0.002 | 0.300 (0.078, 0.522) | 0.004 |
| ANCOVA | 0.0004 | − 0.024 (− 0.219 to 0.171) | 1.0 | 0.286 (0.083 to 0.488) | 0.002 | 0.311 (0.104 to 0.516) | 0.001 | |||
| Change in lumbar spine aBMD g/cm2 | ||||||||||
| ITT | 0.034 (0.047) | 0.031 (0.052) | 0.069 (0.057) | 0.0001 | − 0.002 (− 0.023, 0.019) | 1.0 | 0.036 (0.014, 0.058) | < 0.001 | 0.038 (0.015, 0.060) | < 0.001 |
| Per protocol | 0.034 (0.048) | 0.036 (0.051) | 0.072 (0.055) | 0.0001 | 0.002 (− 0.020, 0.024) | 1.0 | 0.038 (0.015, 0.060) | < 0.001 | 0.036 (0.013, 0.059) | 0.001 |
| ANCOVA | 0.0001 | − 0.001 (− 0.022, 0.021) | 1.0 | 0.036 (0.014, 0.059) | < 0.001 | 0.037 (0.015, 0.060) | < 0.001 | |||
| Change in total body less head aBMD g/cm2 | ||||||||||
| ITT | 0.016 (0.032) | 0.029 (0.034) | 0.040 (0.030) | 0.0001 | 0.014 (0.001, 0.027) | 0.035 | 0.025 (0.011, 0.038) | < 0.001 | 0.011 (− 0.003, 0.025) | 0.1 |
| Per protocol | 0.016 (0.032) | 0.032 (0.034) | 0.041 (0.030) | 0.0001 | 0.015 (0.002, 0.029) | 0.024 | 0.025 (0.011, 0.039) | < 0.001 | 0.010 (− 0.005, 0.024) | 0.3 |
| Change in total body less head aBMD-Z score | ||||||||||
| ITT | − 0.129 (0.458) | 0.012 (0.505) | 0.169 (0.415) | 0.0016 | 0.141 (− 0.051, 0.333) | 0.2 | 0.298 (0.101, 0.495) | 0.001 | 0.157 (− 0.043, 0.358) | 0.1 |
| Per protocol | − 0.103 (0.448) | 0.067 (0.476) | 0.182 (0.415) | 0.0021 | 0.170 (− 0.024, 0.365) | 0.1 | 0.285 (0.090, 0.481) | 0.002 | 0.115 (− 0.086, 0.317) | 0.5 |
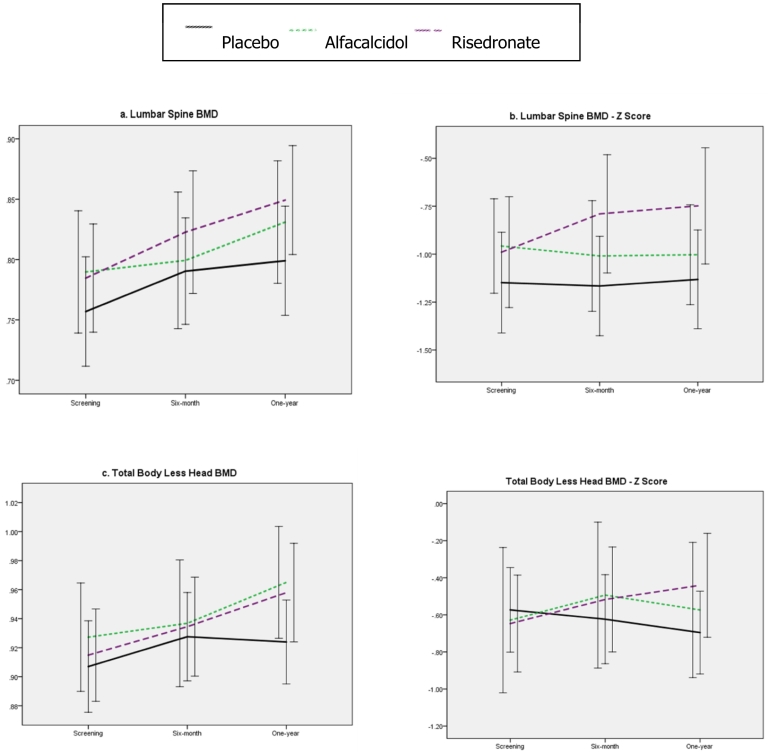
During the year of treatment, LSaBMD z-score was unchanged in the placebo group; − 1.15 to − 1.13; decreased from − 0.96 to − 1.00 in the alfacalcidol group and increased from − 0.99 to − 0.75 in the risedronate group.
LSaBMD and TBLHaBMD increased in all groups, the largest increase being in the risedronate group, the least in the placebo group.
Forest plots for mean (95% CI) change in lumbar spine BMD Score according to treatment group and tanner score and treatment group and steroid dose can be seen in Figs. 1 and 2 in Supplementary data.
The primary analysis using ANOVA showed that there was a statistically significant difference between the groups (p = 0.0007). Highly significant differences were observed between the change in LSaBMD z-scores between the placebo and risedronate groups; 0.274, (95% CI 0.061, 0.487; p < 0.001) and between the risedronate and the alfacalcidol groups; 0.326 (95% CI 0.109, 0.543; p < 0.001) but not between the placebo and alfacalcidol groups; − 0.052 (− 0.257, 0.153). The ANCOVA on LSaBMD z-score at one year, after adjusting for baseline covariates, showed that there is statistically significant between group difference (p = 0.0004) Table 4 shows the mean (95% CI) for the pairwise comparisons post ANCOVA. Fig. 3.
Fig. 3.
a) Lumbar spine BMD, b) lumbar spine BMD Z-score, c) total body less head areal BMD and d) total body less head areal BMD z-scores by treatment group during the course of the study (population: ITT).
3.2. Fracture data
Non-vertebral fracture rates in the placebo, alfacalcidol and risedronate groups were 5.3 (n = 4), 2.8 (n = 2) and 7.2 (n = 5) respectively. There was no statistically significant difference in the fracture rate between the treatment groups (Fisher's exact p = 0.51).
One hundred and eighty-seven patients with pre and post treatment lateral spinal X-rays were scored, using the Genant scoring system. Fifty four patients in the Placebo arm had baseline Genant score of 0 which remained unchanged for all at one year. In the alfacalcidol group, in all of the 52 patients with baseline and one year radiographs the Genant score remained unchanged (50 scored 0, 1 scored 2, 1 scored 3). In the risedronate group, all the 53 patients had a Genant score of 0 at baseline; at one year 50 remained unchanged, 2 had a Genant score of 1 and 1 had a Genant score of 3.
3.3. Biochemical and haematological parameters
There were no statistically significant differences in the biochemical profiles between the three treatment groups at baseline and at 3 months and one year. Twenty-five hydroxy Vitamin D levels were similar in all three groups at baseline and increased in all three groups at 3 months. Alkaline phosphatase increased in all three groups over one year. PTH (ng/l) fell in both the placebo and alfacalcidol groups during the first 3 months from 39.8 (19.9) to 32.6 (20.1) and 34.8 (19.9) to 28.2(17.6) respectively, whilst in the risedronate group it rose from 41 (24.2) to 45.4 (31.9). No change was observed in either calcium or phosphate and disease activity as measured by CRP remained similar between the three groups throughout the year of the study (see Table 5).
Table 5.
Mean (SD) of biochemical parameters by treatment arm (population: ITT).
| Variable | Visit | Placebo | Alfacalcidol | Risedronate |
|---|---|---|---|---|
| Calcium mmol/l | Baseline | 2.4 (0.1) | 2.4 (0.1) | 2.4 (0.1) |
| One year | 2.4 (0.1) | 2.4 (0.1) | 2.4 (0.1) | |
| Phosphate mmol/l | Baseline | 1.4 (0.2) | 1.3 (0.3) | 1.3 (0.2) |
| One year | 1.4 (0.2) | 1.3 (0.3) | 1.3 (0.2) | |
| 25 hydroxy Vitamin D nmol/l |
Baseline | 49.2 (28.5) | 50.2 (26.6) | 44.8 (26.7) |
| 3 montha | 59.9 (24.8) | 67.1 (41.3) | 63.5 (32.8) | |
| PTH ng/l | Baseline | 39.8 (19.9) | 34.8 (19.9) | 41.0 (24.2) |
| 3 month | 32.6 (20.1) | 28.7 (17.6) | 45.4 (31.9) | |
| ALP IU | Baseline | 187.0 (126.6) | 196.1 (133.2) | 179.1 (112.4) |
| One year | 217.8 (158.5) | 233.4 (191.9) | 214.9 (155.8) | |
| CRP mg/l | Baseline | 5.2 (5.8) | 7.8 (11.4) | 7.8 (9.5) |
| One year | 4.8 (5.2) | 6.2 (6.2) | 5.7 (5.7) |
Some parameters were only measured at baseline and 3 months.
3.4. Bone markers
Changes in bone alkaline phosphatase, osteocalcin and crosslaps are shown in Supplementary data Table 2. None of the bone markers predicted the change in BMD at one year.
3.5. Safety
Among 217 patients who were randomised to the trial, 215 patients received at least one dose of study drug and 180 patients experienced at least one adverse event. There was no statistically significant difference between the number of patients experiencing AEs and SAEs between the groups. Higher SAEs were observed in the risedronate group compared to placebo group but only one of these was an adverse reaction i.e. possibly related to the treatment (Table 6). Further details on the types of SAEs are available in Supplementary data Table 3.
Table 6.
Adverse events and serious adverse event summary (safety population).
| Placebo n = 77 | Alfacalcidol n = 71 | Risedronate n = 69 | |
|---|---|---|---|
| Number of patients | |||
| Experiencing an AE | |||
| n | 62 | 59 | 59 |
| Proportion (95% CI) | 0.81 (0.70 to 0.89) | 0.83 (0.72 to 0.91) | 0.86 (0.75 to 0.93) |
| Experiencing an SAE | |||
| n | 18 | 14 | 21 |
| Proportion (95% CI) | 0.23 (0.14 to 0.34) | 0.20 (0.11 to 0.31) | 0.30 (0.20 to 0.43) |
| Number of events | |||
| Number of AEs | 308 | 260 | 292 |
| Proportion (95% CI) | 0.36 (0.33 to 0.39) | 0.30 (0.27 to 0.33) | 0.34 (0.31 to 0.37) |
| Number of ARsa | 17 | 11 | 15 |
| Proportion (95% CI) | 0.40 (0.25 to 0.56) | 0.26 (0.14 to 0.41) | 0.35 (0.21 to 0.51) |
| Number of SAEs | 21 | 22 | 31 |
| Proportion (95% CI) | 0.28 (0.19 to 0.40) | 0.30 (0.20 to 0.41) | 0.42 (0.31 to 0.54) |
| Number SARs | 0 | 2 | 1 |
| Proportion (95% CI) | 0.33 (0.01 to 0.91) | 0.67 (0.09 to 0.99) | 0 (0.0 to 0.71) |
An AE with a definite/probable/possible relationship to the study drug is considered as an AR.
4. Discussion
We have demonstrated that the bisphosphonate risedronate significantly increased LSaBMD z-score in children and young people (CYP) receiving steroids for the treatment of their rheumatological disease, compared to those receiving alfacalcidol or vitamin D and calcium alone. The improvement in LSaBMD z-score for our CYP treated with risedronate was substantially greater than the increases in BMD typically observed in adult studies of glucocorticoid-induced osteoporosis [26]. Our trial was not powered to study the impact of risedronate on fracture rates in CYP; such a trial might require several thousand participants. We observed a 28% increase in BMD z-score in children treated with risedronate compared to a 2% increase and a 10% decrease in CYP treated with placebo or alfacalcidol. Whilst one cannot draw a direct comparison between improvements in adult and CYP BMD T and Z scores, very modest improvements of 6% in BMD in women treated with a bisphosphonate resulted in an almost halving of fractures [27] Furthermore in a study of children with osteogenesis imperfecta treated with bisphosphonates a difference in z-score of 0.387 between the treated and placebo groups was associated with a 47% decrease in fracture rate [28].
This is the first fully powered blinded randomised controlled trial into the prevention and treatment of osteopenia in this population. Two previous RCTs were terminated due to failure to recruit demonstrating the difficulty of undertaking such trials in CYP [29], [30].
We suggest that in association with our observed increase in BMD, fracture risk should also be reduced, even though the observed increase in LSaBMD z-score was less than the hoped-for 0.5.
Many paediatric rheumatologists use alfacalcidol rather than bisphosphonates, as they consider it safer; there was evidence that this prodrug could also improve BMD in adult patients treated with steroids [15], [16]. Our study demonstrates that alfacalcidol has no impact on LSaBMD z-score in children with inflammatory conditions receiving glucocorticoids. Alfacalcidol did result in a significant improvement in TBLHaBMD, but not TBLHaBMD z-score, that is, not after accounting for changes due to age and sex. Alfacalcidol is not without risk; as it bypasses the normal renal regulation, excessive alfacalcidol administration can result in serious hypercalcaemia and organ damage [31].
The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal.
We had hoped that early changes in bone markers might identify those patients who were or were not responding to treatment, allowing timely changes to be made to patient's management. However in our population, bone markers were not able to predict which patients responded best to treatment. Alkaline phosphatase levels increased in all three treatment groups over time as would be expected in growing CYP. The increase in PTH levels in the risedronate group at 3 months is probably due to the effective inhibition of bone resorption which induces a reduction in serum calcium leading to increased parathyroid hormone (PTH) levels.
All therapeutic interventions were well tolerated and the side effect profiles were similar between all three groups. Whilst SAEs in the risedronate group were 7% higher than in the control group there was no difference in the SARs where the reactions were considered to be related to the treatment. SAEs in the risedronate group and were primarily related to disease flares. This study is not large enough to evaluate whether bisphosphonates might induce disease flares. I should be noted that the participants in this trial all had moderate to severe disease, requiring steroids, thus disease flares would be expected.
Avascular necrosis of the mandible has been reported in adults treated with bisphosphonates [32]. We had no such cases and have not identified any in the literature in children.
The long-term effects of bisphosphonates in growing children have as yet not been fully evaluated, although longitudinal cohort studies of children with OI (most often exposed to these drugs) are generally reassuring [33].
5. Conclusion
We have demonstrated that the bisphosphonate risedronate results in statistically and, we believe, clinically meaningful increases in bone mass in both the whole body and the lumbar spine, a site at particular risk for fracture in children with low bone mass in association with inflammatory conditions. The drug was well tolerated with no significant increase in side effects over the comparators. We would advise consideration of risedronate in children and young people with inflammatory conditions receiving steroids, especially those considered at higher risk for fracture.
Acknowledgments
Acknowledgments
Trial Steering committee: Dr. Caroline Dore; Prof Deborah Symmons; Dr. Ann Hall.
DEMEC arc CLINICAL TRIALS COLLABORATION.
(In association with the MRC Clinical Trials Unit, BSR and BOA)
Dr. Ade Adebajo.
Dr. Marwan Bukhari.
Professor Lee Shepstone.
Mike Parker interim analysis NICTU.
Nicola Crabtree for expert analysis and calculation of under – 5 yrs BMDz scores.
Clinical research staff who undertook the clinical trial.
Children and young people and their parents who participated in this study.
There are no competing interests.
EuDRACT No: 2005-003129-23;ISRCTN66814619Office for Research Ethics Committees Northern Ireland Sponsor ID No: 04/MR/111.
Contributorship
- Madeleine Rooney
Study conception Trial design, funding application; patient participation; data analysis; interpretation; paper drafting
- Nick Bishop
Trial design; funding application data analysis; interpretation; paper drafting
- Joyce Davidson
Trial Design; Patient participation; paper drafting
- Michael Beresford
Patient participation; paper drafting
- Clarissa Pilkington
Patient participation; paper drafting
- Janet McDonagh
Patient participation; paper drafting
- Sue Wyatt
Patient participation; paper drafting
- Janet Gardner Medwin
Patient participation; paper drafting
- Rangerag Satyapal
Patient participation; paper drafting
- Jacqi Clinch
Patient participation; paper drafting
- Helen Foster
Patient participation; paper drafting
- Mark Elliott
Radiology evaluation
- Rejina Verghis
Clinical trial statistician and data analyst; paper drafting
We will be happy to share our data with appropriate researchers.
Footnotes
The study was funded by Arthritis Research UK (Versus Arthritis).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.06.004.
Appendix A. Supplementary data
Supplementary material
References
- 1.Prahalad S., Zeft A.S., Pimentel R. Quantification of the familial contribution to juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:2525–2529. doi: 10.1002/art.27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varamos S., Ansell B.M., Reeve J. Vertebral collapse in juvenile chronic arthritis: its relationships with glucocorticoid therapy. Calcif Tissue Int. 1987;41:75–78. doi: 10.1007/BF02555248. [DOI] [PubMed] [Google Scholar]
- 3.Chang J.S., Quinn M.J., Demaziere A., Bulstrode C.J., Francis M.J., Duthrie R.B. Bone resorption by cells isolated from rheumatoid synovium. Ann Rheum Dis. 1992;51:1223–1229. doi: 10.1136/ard.51.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon M.C., White P.H., Raiten D.J. Nutritional status and growth in juvenile rheumatoid arthritis. Semin Arthritis Rheum. 1990;20:97–106. doi: 10.1016/0049-0172(90)90022-8. [DOI] [PubMed] [Google Scholar]
- 5.Rooney M., Davies U.M., Reeve J., Preece M., Ansell B.M., Woo P.M.M. Bone mineral content and bone mineral metabolism: changes after growth hormone treatment in juvenile chronic arthritis. J Rheumatol. 2000;27:41073–41081. [PubMed] [Google Scholar]
- 6.Deodhar A.A., Wolf A.D. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol. 1996;35:309–322. doi: 10.1093/rheumatology/35.4.309. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein B.H., Stobie D., Singsen B.H. Growth retardation in juvenile rheumatoid arthritis. Arthritis Rheum. 1977;20(Suppl,2):212–216. [PubMed] [Google Scholar]
- 8.Kotiani A., Svolainen A. Estimation of central osteopenia in children with chronic polyarthritis treated with glucocorticoids. Pediatrics. 1993;91:1127–1130. [PubMed] [Google Scholar]
- 9.Bianchi M.L., Bardare M. Bone metabolism in juvenile rheumatoid arthritis. J Bone Miner Res. 1990;9:153–162. doi: 10.1016/0169-6009(90)90081-p. [DOI] [PubMed] [Google Scholar]
- 10.Farber H.J., Silveira E.A., Vicere D.R. Oral corticosteroid prescribing for children with asthma in a Medicaid Managed Care Program. Pediatrics. 2017;139(5) doi: 10.1542/peds.2016-4146. [DOI] [PubMed] [Google Scholar]
- 11.van Staa T.P., Cooper C., Leufkens H.G., Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18(5):913–918. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 12.Reed A.M., Haugen M., Pachman L.M., Langman C.B. Repair of osteopenia in children with juvenile rheumatoid arthritis. J Pediatr. 1993;122(5):693–696. doi: 10.1016/s0022-3476(06)80006-5. [DOI] [PubMed] [Google Scholar]
- 13.Buckley L., Guyatt G., Fink H.A., Cannon M., Grossman J., Hansen K.E. 2017 American College of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017;69(8):1095–1110. doi: 10.1002/acr.23279. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook P., Birmingham J., Kelly P., Kempler S., Nguyen T., Pocock N. Prevention of corticosteroid osteoporosis. A comparison of calcium, calcitriol, and calcitonin. N Engl J Med. 1993;328(24):1781–1782. doi: 10.1056/NEJM199306173282404. [DOI] [PubMed] [Google Scholar]
- 15.Reginster J.Y., Kuntz D., Verdickt W., Wouters M., Guillevin L., Menkes C.J. Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporosis Int. 1999;9(1):75–81. doi: 10.1007/s001980050118. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi M.L., Cimaz R., Bardare M., Zulian F., Lepore L., Boncompagni A. 2000 Efficacy and safety of alendronate for the treatment of osteoporosis in diffuse connective tissue diseases in children: a prospective multicenter study. Arth & Rheum. 2000;43(9):1960–1966. doi: 10.1002/1529-0131(200009)43:9<1960::AID-ANR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Rudge S., Hailwood S., Horne A., Lucas J., Wu F., Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology. 2005;44(6):813–818. doi: 10.1093/rheumatology/keh538. [DOI] [PubMed] [Google Scholar]
- 18.Ward L., Tricco A.C., Phuong P., Cranney A., Barrowman N., Gaboury I. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev. 2007;17(4) doi: 10.1002/14651858.CD005324.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayasena A., Atapattu N., Lekamwasam S. Treatment of glucocorticoid-induced low bone mineral density in children: a systematic review. Int J Rheum Dis. 2015;18(3):287–293. doi: 10.1111/1756-185X.12560. [DOI] [PubMed] [Google Scholar]
- 20.Crofton P.M., Evans N., Taylor M.R.H., Holland C.V. Serum crosslaps: paediatric reference intervals from birth to 19 years of age. Clin Chem. 2002;48:671–673. [PubMed] [Google Scholar]
- 21.Crabtree NJ, Oldroyd B, Bishop NJ, et al A paediatric lumbar spine phantom for inter-site and inter-machine cross-calibration. In: Bone 2005 36(5) Suppl 1. S44.
- 22.Cole T.J., Green P.J. Smoothing reference centile curves: the lms method and penalized likelihood. Statist Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 23.Crabtree N.J., Shaw N.J., Bishop N.J., Adams J.E., Mughal M.Z., Arundel P. Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults-the ALPHABET Study. J Bone Miner Res. 2017;32(1):172–180. doi: 10.1002/jbmr.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993; 8:1137–1148. [DOI] [PubMed]
- 25.Saag K.G., Emkey R., Schnitzer T.J., Brown J.P., Hawkins F., Goemaere S. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-induced osteoporosis intervention study group. N Engl J Med. 1998;339(5):292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 26.Black D.M., Cummings S.R., Karpf D.B., Cauley J.A., Thompson D.E., Nevitt M.C. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. Dec 7. [DOI] [PubMed] [Google Scholar]
- 27.Bishop N., Adami S., Ahmed S.F., Antón J., Arundel P., Burren C.P. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9902):1424–1432. doi: 10.1016/S0140-6736(13)61091-0. Oct 26. [DOI] [PubMed] [Google Scholar]
- 28.Green R P Washington University Medical School-St. Louis Children's Hospital St. Louis, Missouri, USA Clinical Trials.gov identifier NCT00022841.
- 29.Brown J., Zacharin M. Attempted randomized controlled trial of pamidronate versus calcium and calcitriol supplements for management of steroid-induced osteoporosis in children and adolescents. J Paediatr Child Health. 2005;41:580–582. doi: 10.1111/j.1440-1754.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 30.Savage M. Complications with reformulated one-alpha vit D. BMJ. 2001;322(7289):799. [PMC free article] [PubMed] [Google Scholar]
- 31.Park W., Kim N.K., Kim M.Y., Rhee Y.M., Kim H.J. Osteonecrosis of the jaw induced by oral administration of bisphosphonates in Asian population: five cases. Osteoporosis Int. 2010;21:527–533. doi: 10.1007/s00198-009-0973-3. [DOI] [PubMed] [Google Scholar]
- 32.Biggin A., Munns C.F. Long-term bisphosphonate therapy in osteogenesis imperfecta. Curr Osteoporos Rep. 2017;15(5):412–418. doi: 10.1007/s11914-017-0401-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material