Fig. 3.
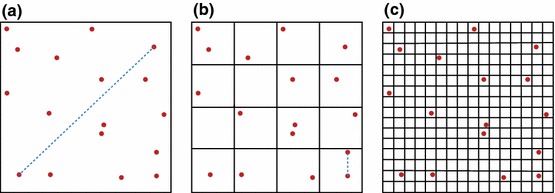
The rationale behind dividing a volume into subvolumes. a The principle of mass action claims that every reacting pair of molecules is equally likely to react in any time period, including pairs which might be very distant (blue dotted line). b One solution is to divide the volume into M subvolumes, and only allow molecules in the same subvolume to react, so reacting pairs are now close together (blue dotted line). c If M is too large, then there will very rarely be pairs in the same subvolume, and bimolecular reactions will tend not to happen at all
