Fig. 4.
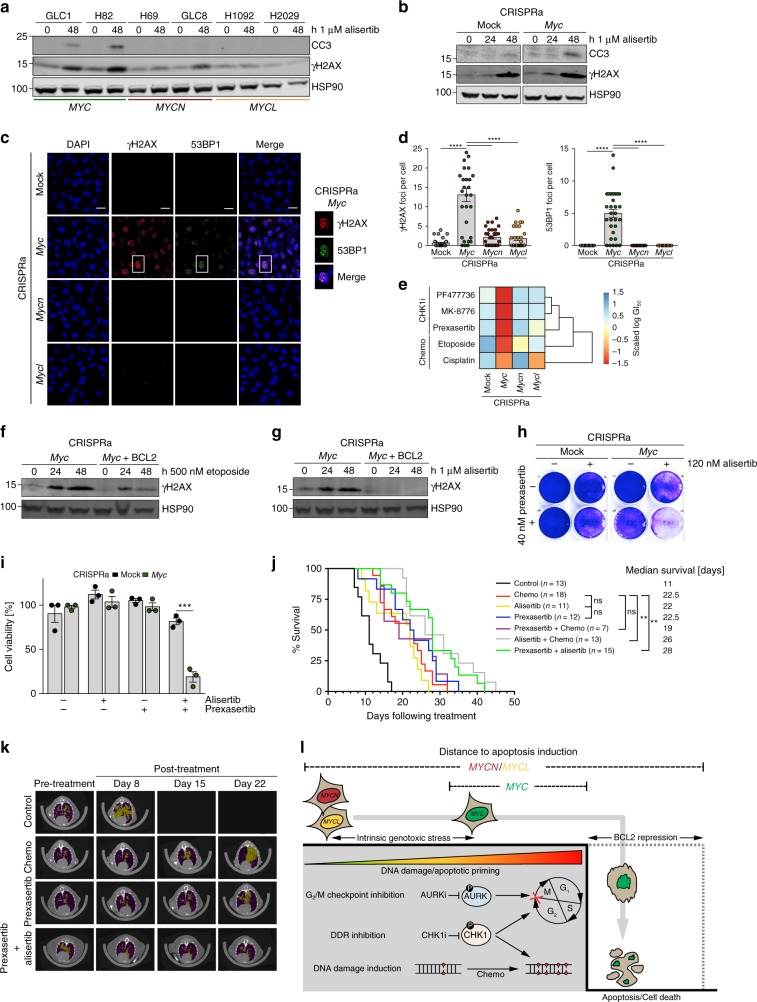
MYC triggers a druggable DNA-damage response (DDR) in vivo. a, b Western blot of cleaved caspase 3 (CC3) and γH2AX in MYC-variant-amplified human small cell lung cancer (SCLC) cell lines (n = 6) (a) or Myc-activated CRISPRa cells (b) treated with alisertib for the indicated times. HSP90 was used as a loading control. c Representative images of immunofluorescence (IF) experiments of Myc paralog-activated CRISPRa cells showing DAPI (DNA), γH2AX (DDR activation), and 53BP1 (DNA double-strand breaks) staining (Scale bar: 20 µm). d Quantification of c showing mean number of γH2AX (top) and 53BP1 (bottom) foci per cell (n = 30). Error bars indicate mean ± SEM. One-way analysis of variance, ****p < 0.0001. e Heatmap displaying sensitivity (scaled log(GI50)) of Myc paralog-activated CRISPRa cells treated with CHK1 inhibitors (MK8776, PF477736, prexasertib) or chemotherapeutics (etoposide, cisplatin) for 96 h (n = 3). f, g Western blot of γH2AX in Myc-activated CRISPRa cells ± BCL2 overexpression treated with etoposide (g) and alisertib (h). HSP90 was used as a loading control. h Crystal violet assay of control and Myc-activated CRISPRa cells upon treatment with 120 nM alisertib, 40 nM prexasertib, and combined treatment for 96 h. i Viability of mock control and Myc-activated CRISPRa cells upon treatment with 120 nM alisertib, 40 nM prexasertib, and combined treatment for 96 h (n = 3). Error bars indicate mean ± SEM. Two-tailed unpaired t tests, ***p < 0.001. j Survival analysis of RPM mice bearing MYC-driven SCLC treated with vehicle control (phosphate-buffered saline (PBS), n = 13), chemotherapy (cisplatin/etoposide, n = 18), Aurora Kinase (AURK) inhibitor alisertib (n = 11), checkpoint kinase 1 (CHK1) inhibitor prexasertib (n = 12), prexasertib+chemotherapy (n = 7), alisertib+chemotherapy (n = 13), and prexasertib+alisertib (n = 15). Log-rank (Mantel–Cox) test, **p < 0.009. k Representative micro-computed tomographic images of RPM mice pre-treatment and after treatment with vehicle control (PBS), chemotherapy (cisplatin/etoposide), CHK1 inhibitor prexasertib, and prexasertib combined with AURK inhibitor alisertib. Tumors are colored in yellow, air space in purple. l Model of MYC paralog-dependent apoptotic priming and vulnerabilities in SCLC. Source data are provided as a Source Data file