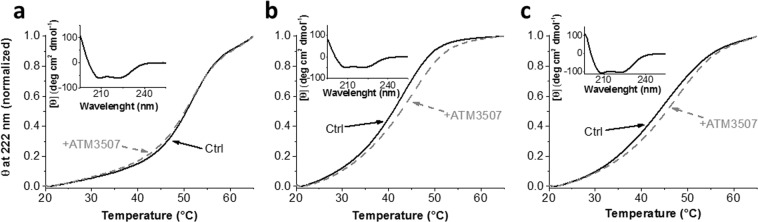
Figure 2.
ATM-3507 binds specifically to the C-terminus of Tpm3.1 and the overlap junction. (a) Normalized unfolding profile of N-terminal 82AA_Tpm3.1 construct (20 µM) in the absence (black solid line; n = 3) and the presence (gray dashed line; n = 3) of ATM-3507 (100 µM). (b,c) Normalized unfolding profiles of the C-terminal 109AA_Tpm3.1 construct (20 µM) and the mixture of N- and C-terminal Tpm3.1 constructs (both at 20 µM), respectively. Black solid lines represent controls in the presence of 1% acetonitrile [(b) n = 3; (c) n = 2] and gray dashed lines are protein samples (20 µM) containing 100 µM of ATM-3507 [(b) n = 6; (c) n = 6]. The CD spectra of all constructs in the inset of individual panels show the expected α-helical protein profile and were measured at 37 °C prior to temperature ramping experiments. Buffer conditions for both spectra scan and thermal unfolding were 10 mM NaH2 PO4 pH 7, 150 mM NaCl, 75 µM TCEP, 1% (v/v) acetonitrile.