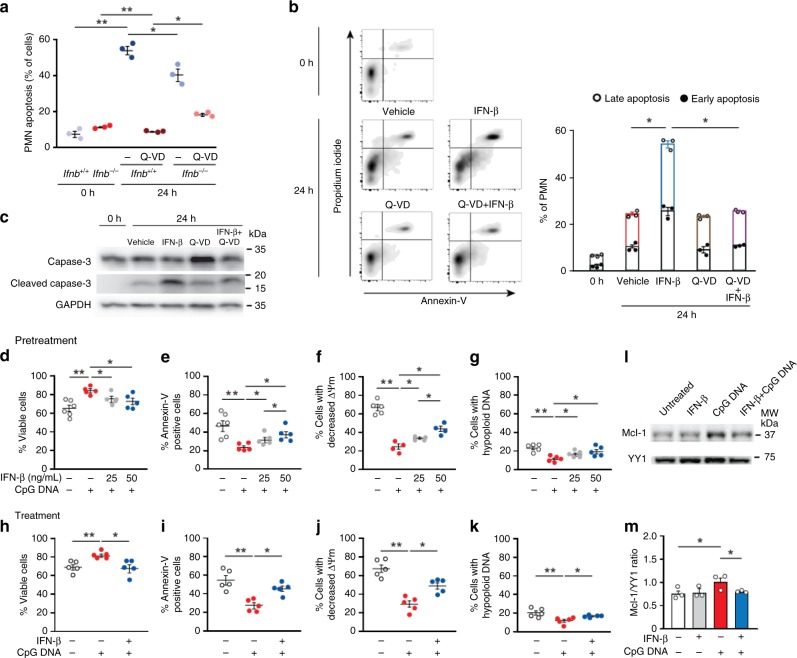
Fig. 4.
IFN-β promotes apoptosis in mouse and human neutrophils. a Peritoneal PMN were recovered from male Ifnb+/+ or Ifnb−/− mice at 4 h PPI and stained immediately with annexin-V and propidium iodide to assess apoptosis and cell viability, respectively with flow cytometry. Alternatively, cells were cultured with or without the pan-caspase inhibitor Q-VD (10 μM) for 24 h and then assessed for apoptosis. Results are mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 (Tukey’s HSD). b, c Peritoneal PMN were recovered from Ifnb+/+ mice 24 h PPI and cultured ex vivo with IFN-β (20 ng/ml) and/or Q-VD (10 µM) for 24 h. Apoptosis was evaluated as above. In some experiments, PMN lysates were prepared after 6 h culture and immunoblotted for cleaved (active) caspase-3. Results are representatives for three independent experiments. *P < 0.05 (Tukey’s HSD). d–g Human PMN (5 × 106 cells/ml) were pretreated with human recombinant IFN-β (25–50 ng/ml) for 10 min and then challenged with CpG DNA (1.6 μg/ml) or (h–k) first challenged with CpG DNA (1.6 μg/ml) and then treated with IFN-β (50 ng/ml) at 60 min post-CpG DNA. Cell viability (d, h), annexin-V staining (e, i), mitochondrial transmembrane potential (ΔΨm; CMXRos staining, f, j) and nuclear DNA content (g, k) were analyzed after culturing neutrophils for 24 h with CpG DNA. Results are mean ± SEM of 5 experiments with different blood donors. *P < 0.05, **P < 0.01 (Dunn’s multiple contrast hypothesis test). l, m Human PMN lysates, prepared following 4 h culture with IFN-β (50 ng/ml) with or without CpG DNA, were immunoblotted for Mcl-1 or the ubiquitous transcription factor YY1 as a loading control. Representative blots (l) and densitometry analyses (m) for three independent experiments. *P < 0.05, **P < 0.01 (Dunn’s multiple contrast hypothesis test). Source data are provided as a Source Data file