Abstract
Background
Compromised neurocognition is a core feature of schizophrenia. With increasing studies researching cognitive function of Chinese patients with first-episode schizophrenia (FES) using MATRICS Consensus Cognitive Battery (MCCB), it is not clear about the level and pattern of cognitive impairment among this population.
Aim
To provide a meta-analysis systematically analysing studies of neurocognitive function using MCCB in Chinese patients with FES.
Methods
An independent literature search of both Chinese and English databases up to 13 March 2019 was conducted by two reviewers. Standardised mean difference (SMD) was calculated using the random effects model to evaluate the effect size.
Results
56 studies (FES=3167, healthy controls (HC)=3017) were included and analysed. No study was rated as ‘high quality’ according to Strengthening the Reporting of Observational Studies in Epidemiology. Compared with HCs, Chinese patients with FES showed impairment with large effect size in overall cognition (SMD=−1.60, 95% CI −1.82 to −1.38, I2=67%) and all seven cognitive domains, with the SMD ranging from −0.87 to −1.41. In nine MCCB subtests, patients with FES showed significant difference in Symbol Coding (SMD=−1.90), Trail Making Test (TMT) (SMD=−1.36), Continuous Performance Test-Identical Pairs (SMD=−1.33), Hopkins Verbal Learning Test (SMD=−1.24), Brief Visuospatial Memory Test (SMD=−1.18), Mazes (SMD=−1.16), Category Fluency (SMD=−1.01), Spatial Span (SMD=−0.69) and Mayer-Salovey-Caruso Emotional Intelligence Test (SMD=−0.38).
Conclusions
Our meta-analysis demonstrates that Chinese patients with FES show neurocognitive deficits across all seven MCCB cognitive domains and all nine subtests, particularly in two neurocognitive domains: speed of processing and attention/vigilance, with the least impairment shown in social cognition. Symbol Coding and TMT may be the most sensitive tests to detect cognitive deficit in Chinese patients with FES.
Keywords: meta-analysis as topic, cognitive dysfunction, neuropsychological tests, schizophrenia
Introduction
Cognitive dysfunction is one of the core features of schizophrenia. Studies have shown that the average impairment in multiple domains of cognition in schizophrenia could reach 2 SD below healthy controls (HC).1 Cognitive impairmentis evident in the prodromal stage, in patients with first-episode schizophrenia (FES) and even in patients with clinical high-risk psychosis (CHR-P) or high familial risk, and persist at a relatively stable level over time.2 3 Previous studies suggested that patients with FES and CHR-P show worse performance in the speed of processing,4–7 which may be the reason for deficits in other domains of cognition.8–10 A meta-analysis of longitudinal studies in patients with FES and CHR-P suggested that cognitive impairment in schizophrenia could originate from abnormal neurodevelopment.11 Imaging studies also supported these conclusions from the structural level.12 13 Cognitive impairment is also an important cause of functional disability and an important factor predicting other outcomes that has a significant impact on patients’ quality of life.14
The MATRICS Consensus Cognitive Battery (MCCB) was developed in 2004 to establish a standardised method to measure cognitive function to investigate cognitive-enhancing medications for schizophrenia.15 It comprised 10 tests that assess seven cognitive domains including speed of processing, attention and vigilance, verbal learning, working memory, problem solving, visual learning and social cognition.16
MCCB has been translated into Chinese, and co-norming and standardisation has been done in China, showing sufficient clinical validity and reliability in controls and patients with schizophrenia.17 Our literature search in various English and Chinese databases showed a growing number of studies on cognitive function in FES but a lack of systematic reviews summarising their data, especially for the reports published in Chinese. The systematic reviews done by Dickinson et al8 focused on the domains of memory and the digital coding test, respectively. There have been a few meta-analysis including one by Mesholam-Gately et al18 and a meta-analysis on drug-naive FES19 but they were unable to include sufficient data from the Chinese population due to language limitations.18 The Letter-Number Span (LNS) test was excluded from the original MCCB in China because there are no corresponding alphabets in Chinese. Cognitive measurements are often culturally affected in test batteries, especially in the domain of social cognition, so there may be differences in the profile of neurocognitive impairment in Chinese patients.20 Another meta-analysis done by Zheng et al systematically reviewed cognitive impairment in patients with CHR-P but not with FES in the Chinese population.21 It would be useful to compare our results to see changes in cognition between patients at different points in the course of their psychotic disease, and to further understand the development and course of cognitive impairment in schizophrenia.
In summary, it is necessary to systematically summarise the growing literature on cognitive impairment in Chinese patients with schizophrenia. This meta-analysis aims to review the baseline performance and impairment profile of MCCB of Chinese patients with FES, as well as its correlation with age and years of education. Given the impacts of cultural and social differences on neurocognitive testing, we also hope this meta-analysis could guide us in developing a neurocognitive test battery that is more appropriate for Chinese patients with schizophrenia.
Materials and methods
Search strategy
Figure 1 shows the process of the literature search. We conducted an electronic literature search in both Chinese and English databases up to 13 March 2019. Chinese and English keywords of ‘Schizophrenia’ AND ‘cognition’, AND ‘Neuropsychological Tests’ were used to search Pubmed, Embase, PsycINFO, Cochrane Library, China National Knowledge Infrastructure (CNKI), WANFANG DATA, WEIPU Journal Net(VIP) and Sino Biomedicine Service System (SinoMed). All literatures retrieved were loaded into Endnote X7 and duplicates were deleted. Two authors independently screened the titles and abstracts to identify possible articles for inclusion; reference lists from relevant review articles for additional studies were hand-searched. The two reviewers independently read the full texts and decided which studies to include according to the inclusion and exclusion criteria below. Disagreements between the two authors were resolved by discussions.
Figure 1.
Flowchart of identification of studies. HC, healthy controls; MCCB, MATRICS Consensus Cognitive Battery.
Inclusion and exclusion criteria
The following inclusion criteria were used based on PICOS: Participants (P): Chinese subjects with FES. Intervention (I): not applicable. Comparison (C): HCs. Outcomes (O): primary outcome was the overall cognitive function (composite score of MCCB); secondary outcomes included the seven MCCB cognitive domains: speed of processing, attention, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. Secondary outcomes also included nine subtests: Trail Making Test (TMT), Brief Assessment of Cognition in Schizophrenia-Symbol Coding, Hopkins Verbal Learning Test-Revised (HVLT-R), Wechsler Memory Scale III (WMS-III): Spatial Span, Neuropsychological Assessment Battery (NAB): Mazes, Brief Visuospatial Memory Test-Revised (BVMT-R), Category Fluency: Animal Naming, Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT): Managing Emotions, and Continuous Performance Test-Identical Pairs (CPT-IP). Study design (S): case–control studies. Studies were excluded if (1) the diagnosis was not clear or patients were not in first episode, and (2) they did not compare patients with HCs.
Data extraction
Two authors (YZ and KM) independently checked and extracted the information included in the studies. If a study lacked SD data or not sure if the data they presented were a T score or raw score, the first or the corresponding author was contacted by email for more information. Any inconsistencies were resolved by consensus or involvement of a third reviewer (HZ). We divided the studies included in our systematic review according to whether they used T scores to report their outcomes or the raw scores, since the two types of scores could not be analysed or compared together. We reported our findings in two sections: the neurocognitive performance of Chinese patients with FES in the seven domains covered by the MCCB and their performance in the subtests of MCCB.
Statistical analysis
Data analyses were performed using the R software V.3.5.1, following the recommendation of the Cochrane Handbook for Systematic Reviews. A random effects model was used in all meta-analytical outcomes because heterogeneity was unavoidable in terms of sample size and sampling method of the studies. Since the outcome was reported using different scales in different studies, we used standardised mean differences (SMD) to evaluate the effect size of the meta-analytical results for all the continuous outcomes. We conducted mega-regression to analyse the effect of age and years of education on effect size. We also did sensitivity analysis, if the heterogeneity of effect size was higher than 50%, to identify the study which could account for more than 10% of the heterogeneity. Funnel plot and Egger test were conducted to explore public bias if the study number of an outcome was more than 10.
Assessment of study quality
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)22 and Grading of Recommendations Assessment, Development and Evaluation (GRADE)23–26 were used to assess the quality of each study by two authors (YW and HZ) independently.
Results
We retrieved 858 records through literature search, no additional records were added through hand search of relevant reviews. Two hundred and twenty-seven duplicates were removed. Six hundred and thirty-one articles were screened for titles and abstracts and 537 reports were excluded according to the inclusion and exclusion criteria. Ninety-four full texts were examined, we excluded 16 articles because of overlapping data. Twenty studies did not offer meta-analysable data. It was not sure if the MCCB scores were T scores or raw scores in one study and there was a lack of SD data in another study, so we tried to contact the corresponding authors to clarify but got no response. Finally, 56 studies were included in the meta-analysis (figure 1).
Study characteristics
Study and patient characteristics are summarised in table 1. All studies were conducted in China (n=56). The mean age of patients ranged from 13.8 to 32.39 and the mean age of healthy controls ranged from 13.79 to 44.7. The criteria used for FES included the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (33, 58.92%), the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)(1,1.8%);the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (17, 30.36%), and the Chinese Classification of Mental Disorders, Third Edition (5, 8.93%); one study used DSM-IV or ICD-10 and three studies asked for Positive and Negative Syndrome Scale scores ≥60 in addition.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | N (nFES/nHC) |
Subjects with FES | Healthy controls | ||||||
| Diagnostic criteria | Male (%) | Age (years) | Education (years) | Duration (months) | Male (%) | Age (years) | Education (years) | ||
| Liang et al75 | 32 (12/20) | ICD-10 | 33.3 | 22.00 | 13.4 (1.9) | 7.0 | 35.00 | 23.50 | 12.5 (3.1) |
| Jiang et al45 | 46 (24/22) | ICD-10 | 45.8 | 15.3 (2.5) | 7.2 (3.9) | 6.0 | 54.55 | 14.8 (2.9) | 8.3 (2.2) |
| Li et al84 | 93 (48/45) | CCMD-3 | 62.5 | 25.9 (9.3) | 10.6 (2.6) | NR | 57.78 | 27.2 (11.9) | 11.3 (3.6) |
| Ai and Chen46 | 122 (60/62) | CCMD-3 | 65.0 | 20.8 (7.9) | NR | NR | 61.29 | NR | NR |
| Chen et al47 | 151 (79/72) | DSM-IV | NR | 13.8 (1.45) | NR | 4.16 (1.86) | 41.67 | 13.79 (1.71) | NR |
| Liu et al48 | 240 (120/120) | CCMD-3 | 50.0 | 23 (10.2) | 12.3 (3.1) | 8 (7.4) | 51.67 | 22 (9.8) | 14.7 (2.6) |
| Yang49 | 204 (102/102) | DSM-IV | 62.7 | 23.8 (3.4) | NR | NR | 56.86 | 23.5 (3.1) | NR |
| Shi et al27 | 60 (37/23) | ICD-10 | 48.6 | 15.35 (1.53) | NR | NR | 39.13 | 15.22 (1.53) | NR |
| Zhao et al66 | 57 (20/37) | ICD-10 | 55.0 | 21.45 (6.6) | 10.95 (2.50) | NR | 59.46 | 20.41 (6.126) | 12.57 (2.41) |
| Xiao et al85 | 60 (30/30) | DSM-IV | 50.0 | 23.5 (2.9) | 12.3 (3.1) | NR | 50.00 | 23.25 (4.21) | 12.6 (2.03) |
| Chen et al28 | 99 (49/50) | DSM-IV | 49.0 | 26.7 (8.5) | 12.3 (1.45) | 31.3 (14.1) | 48.00 | 32.6 (9.3) | 12.1 (1.8) |
| Yao and Hu30 | 158 (83/75) | ICD-10 | 77.1 | 23.15 (3.87) | 12.7 (1.95) | 6.48 (1.32) | 73.33 | 25.23 (7.55) | 15.21 (2.62) |
| Hu et al50 | 154 (92/62) | DSM-IV | 65.2 | 21.19 (3.39) | 11(2) | NR | 59.68 | 21.89 (2.86) | 10.83 (1.49) |
| Zhang et al67 | 60 (30/30) | DSM-IV | 50.0 | 22.8 (4.2) | 12.4 (1.5) | NR | 50.00 | 23.8 (4.0) | 12.5 (1.8) |
| Liu et al52 | 155 (75/80) | DSM-IV | 54.7 | 23(3) | 12.6 (2.8) | 9 (11) | 60.00 | 23(3) | 14(2) |
| Zhang et al67 | 54 (25/29) | DSM-IV | 44.0 | 15.3 (1.7) | 8.7 (1.7) | 5.6 (4.5) | 44.83 | 15.5 (1.5) | 8.6 (1.5) |
| He et al53 | 148 (73/75) | DSM-IV | 58.9 | 24.05 (3.52) | 14.52 (3.16) | NR | 53.33 | 24.12 (3.48) | 14.38 (3.09) |
| Han et al43 | 82 (42/40) | DSM-IV | 28.6 | 20.5 (3.4) | 12.6 (2.6) | NR | 37.50 | 20.2 (1.9) | 13.0 (1.3) |
| Yang et al54 | 133 (79/54) | CCMD-3 | 39.2 | 32.39 (11.2) | 12.68 (3.06) | NR | 27.78 | 32.24 (2.16) | 13.54 (2.16) |
| Zhang et al55 | 68 (41/27) | DSM-IV | 46.3 | 25(7) | 13.2 (2.6) | NR | 55.56 | 26(5) | 13.9 (3.3) |
| Chen et al56 | 102 (52/50) | ICD-10 | 55.8 | 27.23 (9.36) | 13.13 (3.21) | NR | 52.00 | 28.35 (8.52) | 12.67 (2.94) |
| Ma et al57 | 97 (52/45) | DSM-IV | 42.3 | NR | NR | NR | 53.33 | NR | NR |
| Qi59 | 80 (40/40) | DSM-IV | NR | NR | NR | NR | NR | NR | NR |
| Chen et al56 | 60 (30/30) | ICD-10 | 53.3 | 23.85 (4.13) | 10.92 (1.98) | NR | 50.00 | 29.94 (4.08) | 11.63 (1.89) |
| Wei41 | 120 (60/60) | DSM-IV | 63.3 | 22.82 (6.56) | 11.67 (2.83) | NR | 56.67 | 20.97 (5.33) | 11.82 (2.45) |
| Chen et al47 | 210 (145/65) | DSM-IV | 51.7 | 28.5 (9.3) | 13 (3.4) | 18.5 (17.5) | 53.85 | 27.6 (7.4) | 12.6 (2.9) |
| Huang et al31 | 101 (58/43) | ICD-10 | 50.0 | 22.66 (7.64) | 11.41 (2.73) | 15.14 (20.01) | 37.21 | 23.07 (7.49) | 12.65 (3.81) |
| Wu et al32 | 203 (79/124) | DSM-IV | 54.4 | 25.7 (7.8) | 12.7 (3.2) | NR | 52.42 | 44.7 (8.8) | 11.8 (3.4) |
| Zeng et al33 | 116 (55/61) | DSM-IV | 40.0 | 25 (6.36) | 12.65 (2.89) | NR | 45.90 | 25.33 (6.27) | 12.74 (2.77) |
| An et al34 | 60 (30/30) | DSM-IV | NR | NR | NR | NR | NR | NR | NR |
| Chan et al61 | 138 (78/60) | DSM-IV | 62.8 | 28.5 (9.8) | 10.8 (2.5) | 8.2 (14.5) | 31.67 | 28.5 (9.8) | 10.8±2.5 |
| Chen et al35 | 132 (102/30) | DSM-IV | 47.1 | 27.37 (8.85) | 12.53 (3.85) | NR | 46.67 | 26.9 (5.2) | 12.8 (3.8) |
| Guo et al62 | 92 (51/41) | DSM-IV | 64.7 | 22.5 (4.1) | 11.4 (3.3) | 8.4 (6.8) | 80.49 | 22.8 (3.9) | 11.9 (2.7) |
| He et al63 | 152 (80/72) | DSM-IV | 66.3 | NR | NR | NR | NR | NR | NR |
| Hou et al64 | 80 (40/40) | ICD-10 | 60.0 | 26.4 (6.5) | 8.6 (2) | NR | 47.50 | 24.4±5.1 | 10.9 (2.2) |
| Hu et al50 | 112 (56/62) | CCMD-3 | 66.1 | 21.19 (3.39) | 10.89 (1.76) | 10.18 (6.76) | 67.74 | 22 (4) | 11 (1) |
| Wang et al40 | 80 (40/40) | DSM-IV | 43.9 | 23.15 (7.52) | 11.82 (3.84) | NR | 47.63 | 34.54 (3.21) | 10.56 (3.80) |
| Chen et al36 | 78 (42/36) | DSM-IV | 42.8 | 25.21 (6.20) | 12.22 (2.76) | 16.1 (5.4) | 58.30 | 26.47 (4.40) | 14.17 (2.10) |
| Liu et al37 | 116 (44/72) | DSM-IV | 70.5 | 23.5 (4.4) | 11.9 (3.2) | NR | 59.70 | 24.0 (2.9) | 16.0 (2.3) |
| Dong76 | 72 (42/30) | ICD-10 | 61.9 | 27.02 (8.35) | 11.43 (2.68) | NR | 53.30 | 29.23 (5.52) | 12.23 (1.79) |
| Fu68 [68 | 60 (30/30) | DSM-IV/ICD-10 | 43.3 | 23.03 (3.64) | 11.83 (3.15) | 11.23 (5.47) | 40.00 | 24.90 (3.83) | 12.17 (2.73) |
| Ge et al83 | 60 (30/30) | ICD-10 | 53.3 | 25.23 (4.17) | 13.73 (2.18) | 6.49 (2.54) | NR | NR | NR |
| Hao et al38 | 60 (30/30) | ICD-10 | 56.7 | 15.41 (1.96) | NR | NR | 56.70 | 15.57 (1.25) | NR |
| Hu et al72 | 80 (42/38) | DSM-IV-TR | 64.3 | 24.9 (4.8) | 10.5 (2.8) | 8.4 (2.6) | 65.80 | 24.8 (4.6) | 11.1 (2.9) |
| Liu39 | 192 (142/50) | DSM-5 and PANSS ≥60 | 49.3 | 23.94 (5.5) | 12.24 (2.88) | 6.27 (3.83) | 52.00 | 23.7 (4.9) | 12.7 (3.6) |
| Yu69 [69 | 114 (55/59) | ICD-10 | 52.7 | 20.91 (4.87) | 12 (6) | 2 (11) | 37.30 | 22.34 (4.06) | 12 (4) |
| Zhang et al42 | 83 (38/45) | ICD-10 | NR | 25.2 (6.0) | 10.1 (2.7) | NR | NR | 25.2 (5.3) | 10.1 (3.0) |
| Zhang et al42 | 64 (28/38) | DSM-IV | 50.0 | 21.9 (4.0) | 12.8 (2.6) | NR | 42.10 | 24.1 (4.4) | 13.9 (2.6) |
| Zhang44 | 61 (32/29) | DSM-IV | 68.8 | 22.7 (4.0) | 13.1 (2.4) | 10.3 (8.9) | 58.60 | 22.1 (3.6) | 13.8 (2.8) |
| Zhao81 | 109 (50/59) | ICD-10 and PANSS ≥60 | NR | NR | NR | NR | NR | NR | NR |
| Zhou77 | 186 (93/93) | ICD-10 | 33.3 | 25.5 (6.2) | 12.0 (2.2) | 10 (median) | 23.70 | 27.6 (2.7) | 12.6 (1.4) |
| Liang et al70 | 293 (98/195) | DSM-IV | 43.9 | 23.29 (6.79) | 11.80 (2.66) | 19.83 (28.81) | 51.30 | 23.10 (5.45) | 12.18 (2.91) |
| Zhou et al79 | 98 (47/51) | DSM-IV | 59.6 | 25.5 (6.5) | 14.1 (1.8) | 12.8 (11.7) | 42.90 | 24.3 (4.7) | 16.1 (2.6) |
| Zhou et al80 | 49 (32/17) | DSM-IV | 59.4 | 26.2 (8.1) | 13.5 (2.2) | NR | 76.50 | 25.5 (5.6) | 12.6 (2.3) |
| Zhou et al78 | 93 (51/42) | DSM-IV | 64.7 | 25.4 (6.8) | 13.7 (2.2) | 13.2 (11.7) | 42.90 | 24.3 (4.7) | 16.1 (2.6) |
| Wang.40 | 205 (125/80) | DSM-IV and PANSS ≥60 | 49.0 | 23 (7) | 12 (3) | 6 (3) | 52.00 | 24 (4) | 13 (3) |
| Sum | 6184 (3220/2972) | ||||||||
CCMD-3, Chinese Classification of Mental Disorders, Third Edition; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; FES, first-episode schizophrenia; HC, health control;ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision; NR, not reported; PANSS, Positive and Negative Syndrome Scale.
The STROBE score ranges from 0 to 22. Among the included 56 articles (online supplementary table A1), the highest score is 18, and the lowest is 1, with a mean (SD) of 11.41 (4.21). No study was considered of high quality. The main problems of most included studies are: (1) did not describe the setting, location and relevant dates, including periods of recruitment, exposure and data collection; (2) did not describe the aptitude of evaluators and consistencies among evaluators; (3) did not describe any efforts to address potential sources of bias; and (4) did not explain how the sample sizes were arrived at. The overall quality levels of 17 meta-analytical outcomes are evaluated as ‘low’ (5.9%, 1/17), ‘moderate’ (29.4%, 5/17) and ‘high’ (64.7%, 11/17) using the GRADE approach (table 2).
Table 2.
GRADE analyses: neurocognitive dysfunction assessed by MCCB in subjects at clinical high risk for psychosis
| Meta-analytical outcomes | Studies (n) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Large effect | Overall quality of evidence* |
| Composite score | 11 (1427) | No | Serious† | No | No | Serious | Large‡ | +/+/+/−/ Moderate |
| Speed of processing | 7 (966) | No | Serious† | No | No | Serious | Large‡ | +/+/+/−/ Moderate |
| Attention/vigilance | 11 (1487) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| Working memory | 11 (1487) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| Verbal learning | 12 (1569) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| Visual learning | 12 (1569) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| Problem solving | 11 (1487) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| Social cognition | 12 (1570) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| TMT | 30 (3391) | No | Serious† | No | No | Serious | Large‡ | +/+/+/−/ Moderate |
| Symbol Coding | 26 (2855) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| HVLT-R | 27 (2747) | No | Serious† | No | No | Serious | Large‡ | +/+/+/−/ Moderate |
| WMS-III-SS | 17 (1957) | No | Serious† | No | No | No | No | +/+/+/−/ Moderate |
| Mazes | 10 (1078) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| BVMT-R | 22 (2462) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| Fluency | 19 (1933) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
| MSCEIT | 6 (706) | No | Serious† | No | No | Serious | No | +/+/−/−/ Low |
| CPT-IP | 11 (945) | No | Serious† | No | No | No | Large‡ | +/+/+/+/ High |
*GRADE Working Group grades of evidence: high quality=further research is very unlikely to change our confidence in the estimate of effect; moderate quality=further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality=further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality=we are very uncertain about the estimate.
†All studies reported having a serious inconsistency had I2 >50%.
‡Studies with large effects provided increased quality of evidence. Large effects=standard mean differences less than −0.8.
BVMT-R, Brief Visuospatial Memory Test-Revised; CPT-IP, Continuous Performance Test-Identical Pair; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HVLT-R, Hopkins Verbal Learning Test-Revised; MCCB, MATRICS Consensus Cognitive Battery; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; TMT, Trail Making Test; WMS-III-SS, Wechsler Memory Scale III: Spatial Span.
gpsych-2018-100043supp001.docx (438.6KB, docx)
MCCB cognitive domain score comparison between patients with FES and HCs
MCCB composite score
Pooling data from 12 studies that contained MCCB composite scores of patients with FES and HCs (FES=788, HC=639),27–38 the MCCB composite score was significantly lower in patients with FES than controls (SMD=−1.60, 95% CI −1.82 to −1.38, I2=67%) (figures 2 and 3). Sensitivity analysis found that one study33 contributed to the heterogeneity of effect size most, and I2 decreased to zero when omitting this study.
Figure 2.
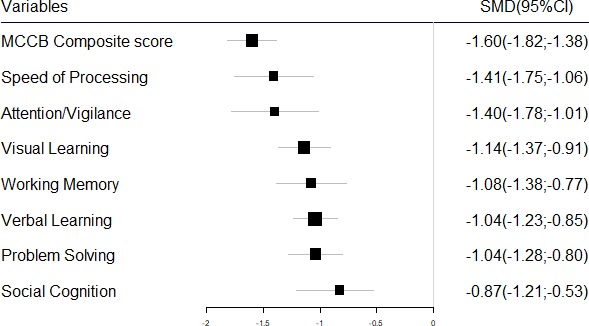
MCCB cognitive domain score comparison between patients with first-episode schizophrenia (FES) and healthy controls. MCCB, MATRICS Consensus Cognitive Battery; SMD, standardised mean difference.
Figure 3.
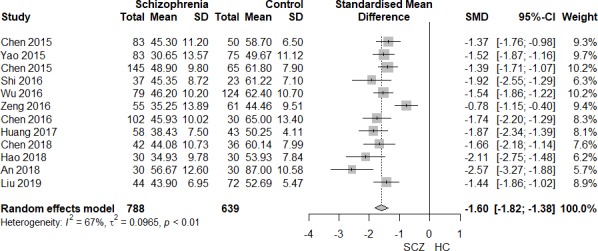
MATRICS Consensus Cognitive Battery (MCCB) composite score comparison between patients with first-episode schizophrenia (FES) and HC. HC, healthy control; SCZ, schizophrenia; SMD, standardised mean difference.
Speed of processing
Pooling data from seven studies that contained speed of processing T scores of patients with FES and HCs (FES=549, HC=417),30 31 33 36 37 39 40 the speed of processing domain score was significantly lower in the patients with FES than the controls (SMD=−1.41, 95% CI −1.75 to −1.06, I2=82%) (online supplementary figure A1; figure 2). Sensitivity analysis found that I2 decreased to 67.5% when omitting the study39 that contributed most to the heterogeneity of the effect size.
Attention/vigilance
Pooling data from 11 studies that contained attention and vigilance T scores of patients with FES and HCs (FES=818, HC=669),30–33 35–37 39–42 the attention and vigilance domain score was significantly lower in the patients with FES than the controls (SMD=−1.40, 95% CI −1.78 to −1.01, I2=90%) (online supplementary figure A2; figure 2). Sensitivity analysis found I2 decreased to 75.2% when omitting the study39 that contributed most to the heterogeneity of the effect size.
Working memory
Pooling data from 11 studies that contained working memory T scores of patients with FES and HCs (FES=818, HC=669),30–33 35–37 39–42 the working memory domain score was significantly lower in the patients with FES than the controls (SMD=−1.08, 95% CI −1.38 to −0.77, I2=86%) (online supplementary figure A3; figure 2). Sensitivity analysis found I2 decreased to 73.9% when omitting the study39 that contributed most to the heterogeneity of the effect size.
Verbal learning
Pooling data from 12 studies that contained verbal learning scores of patients with FES and HCs (FES=860, HC=709),30–33 35–37 39–43 the verbal learning domain score was significantly lower in the patients with FES than the controls (SMD=−1.04, 95% CI −1.23 to −0.85, I2=66%) (online supplementary figure A4; figure 2). Sensitivity analysis found I2 decreased to 33.5% when omitting the study39 that contributed most to the heterogeneity of the effect size.
Visual learning
Pooling data from 12 studies that contained visual learning scores of patients with FES and HCs (FES=860, HC=709),30–33 35–37 39–43 the visual learning domain score was significantly lower in the patients with FES than the controls (SMD=−1.14, 95% CI −1.37 to −0.91, I2=77%) (online supplementary figure A5; figure 2). Sensitivity analysis found I2 decreased to 63.3% when omitting the study39 that contributed most to the heterogeneity of the effect size.
Problem solving
Pooling data from 11 studies that contained problem solving scores of patients with FES and HCs (FES=818, HC=669),30–33 35–37 39–42 the problem solving domain score was significantly lower in the patients with FES than the controls (SMD=−1.04, 95% CI −1.28 to −0.80, I2=77%) (online supplementary figure A6; figure 2). Sensitivity analysis found I2 did not decrease much when omitting any study.
Social cognition
Pooling data from 12 studies that contained social cognition scores of patients with FES and HCs (FES=856, HC=714),30–33 35–37 39–42 44 the social cognition domain score was significantly lower in the patients with FES than the controls (SMD=−0.87, 95% CI −1.21 to −0.53, I2=90%) (online supplementary figure A7; figure 2). Sensitivity analysis found I2 decreased to 77.0% when omitting the study39 that contributed most to the heterogeneity of the effect size.
MCCB subtest score comparison between patients with FES and HCs
Trail Making Test
Pooling data from 30 studies that contained TMT scores (completion time) of patients with FES and HCs (FES=1663, HC=1650), the TMT completion time was significantly longer in the patients with FES than the controls (SMD=1.36, 95% CI 1.15 to 1.58, I2=89%), which indicated that patients with FES had worse performance in TMT than controls(SMD=-1.36, 95% CI -1.58to -1.15) (online supplementary figure A8; figure 4).27 38 45–72 Sensitivity analysis found I2 did not decrease much when omitting any study.
Figure 4.
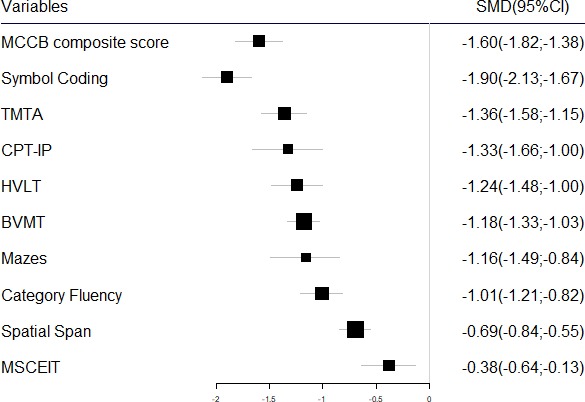
MCCB subtest score comparison between patients with first-episode schizophrenia (FES) and healthy controls. BVMT, Brief Visuospatial Memory Test; CPT, Continuous Performance Test; HVLT, Hopkins Verbal Learning Test; MCCB, MATRICS Consensus Cognitive Battery; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; SMD, standardised mean difference; TMTA, Trail Making Test-A.
Symbol Coding test
Pooling data from 26 studies that contained Symbol Coding subtest raw scores of patients with FES and HCs (FES=1551, HC=1304),27 28 38 45 48 50 52–55 57–60 62–64 66–69 73–77 the Symbol Coding score was significantly lower in the patients with FES than the controls (SMD=−1.90, 95% CI −2.13 to −1.67, I2=84%) (online supplementary figure A9; figure 4). Sensitivity analysis found I2 did not decrease much when omitting any study.
Hopkins Verbal Learning Test-Revised
Pooling data from 27 studies that contained HVLT-R subtest raw scores of patients with FES and HCs (FES=1448, HC=1299),27–29 38 45 46 48–53 55 59 60 62 64–69 75 78–81 the HVLT-R score was significantly lower in the patients with FES than the controls (SMD=−1.24, 95% CI −1.48 to −1.00, I2=87%) (online supplementary figure A10; figure 4). Sensitivity analysis found I2 did not decrease much when omitting any study.
WMS-III: Spatial Span
Pooling data from 17 studies that contained Spatial Span subtest raw scores of patients with FES and HCs (FES=1044, HC=913),27–29 38 43 46 48 50 52 53 62 65–68 75 77 the Spatial Span test score was moderately lower in the patients with FES than the controls (SMD=−0.69, 95% CI −0.84 to −0.55, I2=53%) (online supplementary figure A11; figure 4). Sensitivity analysis found I2 did not decrease much when omitting any study.
NAB: Mazes
Pooling data from 10 studies that contained Mazes subtest raw scores of patients with FES and HCs (FES=590, HC=488),27–29 38 47 66–68 77 81 the Mazes test score was significantly lower in the patients with FES than the controls (SMD=−1.16, 95% CI −1.49 to −0.84, I2=82%) (online supplementary figure A12; figure 4). Sensitivity analysis found I2 decreased to 51.1% when omitting the study77 which contributed most to heterogeneity.
Brief Visuospatial Memory Test-Revised
Pooling data from 22 studies that contained BVMT-R subtest raw scores of patients with FES and HCs (FES=1290, HC=1172),27–29 38 46–53 59 60 62 65–69 75 81 the BVMT-R score was significantly lower in the patients with FES than the controls (SMD=−1.18, 95% CI −1.33 to −1.03, I2=65%) (online supplementary figure A13; figure 4). Sensitivity analysis found I2 did not decrease much when omitting any study.
Category Fluency: Animal Naming (Fluency)
Pooling data from 19 studies that contained Animal Naming Fluency subtest raw scores of patients with FES and HCs (FES=1047, HC=886),27 28 38 50 54 61 62 65–69 71 75–77 81–83 the Category Fluency test score was significantly lower in the patients with FES than the controls (SMD=−1.01, 95% CI −1.21 to −0.82, I2=74%) (online supplementary figure A14; figure 4). Sensitivity analysis found I2 decreased to 62.8% when omitting the study28 that contributed most to the heterogeneity.
MSCEIT: Managing Emotions
Pooling data from six studies that contained MSCEIT subtest raw scores of patients with FES and HCs (FES=401, HC=305),28 29 38 66 68 77 the MSCEIT test raw score was slightly lower in the patients with FES than the controls (SMD=−0.38, 95% CI −0.64 to −0.13, I=60%) (online supplementary figure A15; figure 4). Sensitivity analysis found I2 did not decrease much when omitting any study.
Continuous Performance Test-Identical Pairs
Pooling data from 11 studies that contained CPT-IP subtest raw score of patients with FES and HCs (FES=535, HC=410),27–29 38 59 60 66 68 76 84 85 the CPT-IP score was significantly lower in the patients with FES than the controls (SMD=−1.33, 95% CI −1.66 to −1.00, I2=80%) (online supplementary figure A16; figure 4). Sensitivity analysis found I2 did not decrease much when omitting any study.
Meta-regression
Most studies provided data on age and education years. Meta-regression was conducted to explore the influence of these moderators. It was found that there was a positive correlation between age and SMD of BVMT (coefficient=0.05, z-value=3.18, R2=55.84%), and a small BVMT score difference was found in the younger population group; there was no significant correlation between age and other outcomes. Education years had no significant influence on the study effect size.
Publication bias
Except for the result of speed of processing and MSCEIT, Egger test was conducted to identify publication bias of all other outcomes. Egger test showed there may be potential publication bias in MCCB composite score (t=−2.99, df=10, p=0.013), TMT (t=3.40, df=28, p<0.01) and HVLT-R (t=−3.76, df=25, p<0.01). Funnel plot of MCCB composite score also showed there was asymmetry in the figure (figure 5).
Figure 5.
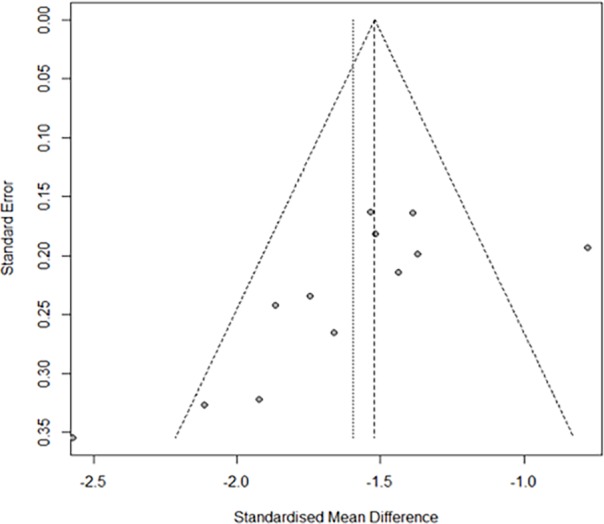
Funnel plot of MATRICS Consensus Cognitive Battery (MCCB) composite score.
Discussion
Main findings
To the best of our knowledge, this was the first meta-analysis that systematically explores neurocognitive function in Chinese patients with FES from the point of view of MCCB results. Compared with HCs, in Chinese patients with FES, significant deficit was found in MCCB composite score (SMD=−1.60, 95% CI −1.82 to −1.38) and all seven cognitive domains. The pooled effect sizes were the greatest in speed of processing and attention, followed by visual learning, working memory, verbal learning, problem solving, social cognition, with SMD between −0.87 and −1.41 in seven cognitive domains. The ranks of SMD of nine MCCB subtest raw scores were as follows: Symbol Coding, TMT, CPT-IP, HVLT, BVMT, Mazes, Category Fluency, Spatial Span, MSCEIT, with pooled effect sizes from −0.38 to −1.90. Age and years of education may have no significant influence on the effect size of studies except for SMD of BVMT, which had a positive correlation with age.
Implications
Our results on the seven cognitive domains and on each MCCB subset both showed the worst impairment in speed of processing and attention/vigilance and the least impairment in social cognition. Although the rank between decline in verbal memory, visual memory, problem solving and working memory is not consistent in the two results, the effect sizes are large and comparable in verbal memory, visual memory and problem solving. A meta-analysis of Xiang et al that focused on patients with CHR-P in China also showed the greatest deficits in the speed of processing and attention/vigilance, while suggesting no statistically significant impairment in social cognition. Our study showed a slightly greater deficit in all of the seven cognitive domains of patients with FES compared with patients with CHR-P. There is also a difference in the order of which working memory, problem solving, visual learning and verbal learning are ranked according to their level of impairment.21 These results could suggest that cognitive impairment continues to progress from the time patients are defined as CHR-P to the start of the first episode, and that different domains of cognition may deteriorate at different rates throughout the course of the disease. This correlates with a meta-analysis on neurocognition in CHR-P young adults who did or did not convert to FES, which concluded that the main difference in the cognitive profile of the two populations is in the domains of working memory and visual learning.86
The findings of our study showed a higher level of impairment in all seven domains of neurocognition among patients with FES compared with the results from the meta-analysis done by Mesholam-Gately et al.18 The effect size for HVLT and WMS-III: Spatial Span falls into the range reported by Mesholam-Gately et al18 while SMDs of Symbol Coding and TMT-A were lower in our study. Interestingly, Chinese patients with FES seem to have less impairment in social cognition. These differences could result from the fact that the other study included research that used cognitive tests not included in the MCCB, and that the tests were grouped differently into various cognitive domains than the MCCB. Furthermore, the Chinese version of MCCB does not have a LNS test that accounts for working memory, which, along with other differences in cultural and language differences, could also lead to discrepancies in the test results across different countries.
Given the potential difference caused by the usage of MCCB in China, it would be interesting to compare the normative data of MCCB with other countries, and to create a neurocognitive test battery that better adapts to the characteristics of Chinese patients with schizophrenia. More research on social cognition in Chinese patients as well as high-quality studies investigating the role of medications and other treatments such as electroconvulsive therapy and transcranial magnetic stimulation in improving cognitive function in patients with schizophrenia are needed. Further studies on longitudinal change in neurocognitive decline in patients with schizophrenia would be helpful in understanding the mechanisms and finding new therapeutic targets. Being the first systematic review on previous literature regarding Chinese patients with FES, this study provides a summary and serves as a basis for future research on this topic.
Strengths and limitations of this study
The main strength of this meta-analysis was that it included studies with MCCB domain score and studies providing MCCB subset raw score, which would enlarge the sample size and enhance certainty of the results. However, the limitations of this study should also be acknowledged. First, among all studies included, no literature was rated as high quality according to STROBE, but most outcomes were rated as high quality using GRADE; the potential reason may be that STROBE and GRADE assess different aspects. The studies we included were cross-sectional studies, we think STROBE shows more precise information. Second, considerable heterogeneity of SMD existed in some results, so we conducted meta-regression to analyse the impact of some possible moderators and sensitivity analysis to find out extreme or abnormal values; by these methods heterogeneity could partially be explained. However, the effects of some potential moderators such as clinical symptoms severity, medication and premorbid IQ were not explored because they were not frequently provided in studies. It is shown in several studies that antipsychotics have a limited effect on cognitive function early in FES.87 88 We originally planned to conduct a subgroup analysis for treated patients and treatment-naive patients. However, we found that very few of the studies included in this review mentioned if the patients had already taken antipsychotics at the time of neurocognitive evaluation. Therefore, the amount of data was not sufficient to perform a subgroup analysis. Third, there was a potential publication bias for the primary outcome MCCB composite score. Considering the limited number of studies, more studies are warranted to confirm our results in the future.
Conclusion
In summary, the Chinese patients with FES performed worse than the HCs in the overall neurocognitive function and all individual cognitive domains. Prominent impairment was particularly seen in Symbol Coding, TMT and CPT-IP, namely in the neurocognitive domains of speed of processing and attention. On the one hand, the result indicates that some neuropsychological tasks are more sensitive and reflect relatively more severe cognitive deficits, which may shed light on the development of new neurocognitive assessment batteries. On the other hand, it reminds us that early effective interventions should be developed and implemented to relieve cognitive damage in Chinese patients with FES.
Biography
Huijuan Zhang obtained a bachelor’s degree in clinical medicine from Shanghai Jiaotong University, Shanghai, China in 2016. At present, she is a medical doctor candidate in Shanghai Mental Health Center, Shanghai, China. Her main research interest includes evidence-based medicine.

Footnotes
Contributors: CL, CS and XY designed the study. CL was assisted by KM in the search of papers. Data extraction was done by CS and YZ. YZ conducted the data analysis. YW, HZ and YH drafted the manuscript. YZ, TZ, JW and CL made critical revisions. All authors approved the final version for publication.
Funding: This study was supported by Shanghai Sailing Program (16YF1416000), Qihang Foundation of Shanghai Mental Health Center (2015-QH-01) and SHSMU-ION Research Centre for Brain Disorders (2017NKX003).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All the relevant data has been presented in Tables and Figures.
References
- 1.Harvey PD, Keefe RSE, Ai C. Cognitive impairment in schizophrenia and implications of atypical neuroleptic treatment. J CNS spectr Medical Journal of Chinese People's Health 1997:225–55. [Google Scholar]
- 2.Bliksted V, Fagerlund B, Weed E, et al. Social cognition and neurocognitive deficits in first-episode schizophrenia. Schizophr Res 2014;153:9–17. 10.1016/j.schres.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 3.Pietrzak RH, Snyder PJ, Jackson CE, et al. Stability of cognitive impairment in chronic schizophrenia over brief and intermediate re-test intervals. Hum. Psychopharmacol. Clin. Exp. 2009;24:113–21. 10.1002/hup.998 [DOI] [PubMed] [Google Scholar]
- 4.Aas M, Dazzan P, Mondelli V, et al. A systematic review of cognitive function in First-Episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front. Psychiatry 2014;4 10.3389/fpsyt.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bora E, Lin A, Wood SJ, et al. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand 2014;130:1–15. 10.1111/acps.12261 [DOI] [PubMed] [Google Scholar]
- 6.McCleery A, Ventura J, Kern RS, et al. Cognitive functioning in first-episode schizophrenia: MATRICS consensus cognitive battery (MCCB) profile of impairment. Schizophr Res 2014;157:33–9. 10.1016/j.schres.2014.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Üçok A, Direk N, Koyuncu A, et al. Cognitive deficits in clinical and familial high risk groups for psychosis are common as in first episode schizophrenia. Schizophr Res 2013;151:265–9. 10.1016/j.schres.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 8.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophreniaJ. Arch Gen Psychiatry 2007;64:532–42. [DOI] [PubMed] [Google Scholar]
- 9.Kenney J, Anderson-Schmidt H, Scanlon C, et al. Cognitive course in first-episode psychosis and clinical correlates: a 4 year longitudinal study using the MATRICS consensus cognitive battery. Schizophr Res 2015;169:101–8. 10.1016/j.schres.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Ojeda N, Peña J, Schretlen DJ, et al. Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophr Res 2012;135:72–8. 10.1016/j.schres.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 11.Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull 2014;40:744–55. 10.1093/schbul/sbt085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprooten E, Papmeyer M, Smyth AM, et al. Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr Res 2013;151:259–64. 10.1016/j.schres.2013.09.024 [DOI] [PubMed] [Google Scholar]
- 13.Yao L, Lui S, Liao Y, et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2013;45:100–6. 10.1016/j.pnpbp.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 14.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?J. Am J Psychiatry 1996;153:321–30. [DOI] [PubMed] [Google Scholar]
- 15.Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 2004;56:301–7. 10.1016/j.biopsych.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 16.Nuechterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res 2004;72:29–39. 10.1016/j.schres.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Shi C, Kang L, Yao S, et al. The MATRICS consensus cognitive battery (MCCB): Co-norming and Standardization in China. Schizophr Res 2015;169:109–15. 10.1016/j.schres.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 2009;23:315–36. 10.1037/a0014708 [DOI] [PubMed] [Google Scholar]
- 19.Fatouros-Bergman H, Cervenka S, Flyckt L, et al. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res 2014;158:156–62. 10.1016/j.schres.2014.06.034 [DOI] [PubMed] [Google Scholar]
- 20.Velligan DI, Rubin M, Fredrick MM, et al. The cultural adaptability of intermediate measures of functional outcome in schizophrenia. Schizophr Bull 2012;38:630–41. 10.1093/schbul/sbq136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W, Zhang Q-E, Cai D-B, et al. Neurocognitive dysfunction in subjects at clinical high risk for psychosis: a meta-analysis. J Psychiatr Res 2018;103:38–45. 10.1016/j.jpsychires.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 22.PLOS Medicine Editors Observational studies: getting clear about transparency. PLoS Med 2014;11:e1001711 10.1371/journal.pmed.1001711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt G, Oxman AD, Akl EA, et al. Grade guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Kunz R, et al. Grade guidelines: 2. framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395–400. 10.1016/j.jclinepi.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 25.Balshem H, Helfand M, Schünemann HJ, et al. Grade guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 27.Shi G, Li R, Liu J, et al. The characteristics of cognitive function impairment in children and adolescents with the first-episode schizophrenia. J Psychiatry 2016;29:429–33. [Google Scholar]
- 28.Chen D, Zhang X, Yang K, et al. Impairment of neurocognitve and Performance-based skills in defict and non-deficit patients with drug-naive first-episode schizophrenia. Chin J Psychiatry 2015;1:23–6. [Google Scholar]
- 29.Chen D, Yang K, Li Y, et al. The study of the asscotiation of impairment of cognition and Performance-based skills with clinical symptoms in drug-naive first-episode schizophrenia patients. Chin J Nerv Ment Dis 2015;1:26–31. [Google Scholar]
- 30.Yao M, Hu J. Application study of MATRICS consensus cognitive battery in first-episode schizophrenia patients. J China Modern Doctor 2015;21:14–17. 20. [Google Scholar]
- 31.Huang M-L, Khoh T-T, Lu S-J, et al. Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Medicine 2017;96:e7228 10.1097/MD.0000000000007228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu JQ, Chen DC, Tan YL, et al. Cognitive impairments in first-episode drug-naive and chronic medicated schizophrenia: MATRICS consensus cognitive battery in a Chinese Han population. Psychiatry Res 2016;238:196–202. 10.1016/j.psychres.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 33.Zeng B, Ardekani BA, Tang Y, et al. Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res 2016;172:1–8. 10.1016/j.schres.2016.01.051 [DOI] [PubMed] [Google Scholar]
- 34.An H, Zhou L, Yu Y, et al. Serum NCAM levels and cognitive deficits in first episode schizophrenia patients versus health controls. Schizophr Res 2018;192:457–8. 10.1016/j.schres.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 35.Chen DC, Du XD, Yin GZ, et al. Impaired glucose tolerance in first-episode drug-naïve patients with schizophrenia: relationships with clinical phenotypes and cognitive deficits. Psychol Med 2016;46:3219–30. 10.1017/S0033291716001902 [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Tian L, Chen N, et al. More dampened monocytic Toll-like receptor 4 response to lipopolysaccharide and its association with cognitive function in Chinese Han first-episode patients with schizophreniaJ. Schizophrenia Research 2018. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wang G, Jin H, et al. Cognitive deficits in subjects at risk for psychosis, first-episode and chronic schizophrenia patients. Psychiatry Research 2019;274:235–42. 10.1016/j.psychres.2019.01.089 [DOI] [PubMed] [Google Scholar]
- 38.Hao R. Characteristics of cognitive impairment in children and adolescents with schizophrenia. J Acta Universitatis Medicinalis Anhui 2018;53. [Google Scholar]
- 39.Liu Y. Combined use of high frequency rTMS and family intervention to improve psychiatric symtoms and cognitive function in first-episode schizophrenia and its mechanism. D. Zhenzhou University 2018. [Google Scholar]
- 40.Wang Y, Zhang P, Yuan X, et al. Effects of oxidative stress on cognitive impairment in first episode schizophrenia. Nat Med J China 2019;99:9–13. [DOI] [PubMed] [Google Scholar]
- 41.Wei Y. The study on the cognition of First-episode schizophrenia and the rTMS intervention. D. Second Military Medical University 2016. [Google Scholar]
- 42.Zhang R, Yan W, Lv L, et al. Regional homogeneity and cognitive funtion in first-episode patients with schizophrenia. J Clin Psychiatry 2018;28:222–5. [Google Scholar]
- 43.Han X, Yang L, Cheng Z, et al. Neurocognitive performance in the patients with first-episode schizophrenia and their indepent first-degree relatives: a cross-sectional studyJ. J Peking University 2010;6:681–6. [PubMed] [Google Scholar]
- 44.Zhang M, Fan F, Tan Y, et al. Resting-state functional connectivity of amygdala and its relationship with emotion regulation in patients with first episode schizophrenia. Chin Men Heal J 2018;32:801–7. [Google Scholar]
- 45.Jiang S, Yang Y, Chen H, et al. The effects of aripiprazole on cognitive function in children and adolescent patients with schizophreniaJ. Jiangxi Medical Journal 2017;52:34–6. [Google Scholar]
- 46.Ai C, Chen S. Comparison of cognitive function in first-episode schizophrenia patients between before and after aripiprazole treatmentJ. Medical Journal of Chinese People's Health 2013;25:14–15. [Google Scholar]
- 47.Chen H, Guo S, Shao R, et al. Behavior characteristics and cognitive function in the first-episode children with or without obsessive-compulsive symtomsJ. Chin J Nerv Ment Dis 2015;41:208–13. [Google Scholar]
- 48.Liu L, He D, Deng X, et al. The survey of impaired neurocognitive function in patients with first-episode schizophreniaJ. Chinese Journal of Practical Nervous Diseases 2014;17:13–16. [Google Scholar]
- 49.Yang S. A primary study of early impairment of cognitive function in first-episode schizophrenia patientsJ. Journal of Clinical and Practice Medicine 2014;13:405–7. [Google Scholar]
- 50.Hu M, Chen J, Li L, et al. Semantic fluency and executive functions as candidate endophenotypes for the early diagnosis of schizophrenia in Han Chinese. Neurosci Lett 2011;502:173–7. 10.1016/j.neulet.2011.07.037 [DOI] [PubMed] [Google Scholar]
- 51.Zhang X. Cognitive function of patients with first-episode schizophrenia and ultra-high risk populationJ. Chinese Journal of Practical Nervous Diseases 2015;23:43–5. [Google Scholar]
- 52.Liu N, Lu Z, Sheng J, et al. The neurocognitive function in patients with first-episode schizophrenia treated in early stageJ. Clin J Psychiatry 2013;2:99–103. [Google Scholar]
- 53.He J, Kong D, Cai F, et al. The changes of neurocognitive function in early stage in patients with First-Episode schizophrenia. J. Progress in Modern Biomedicine 2017;22:4277–80. 4298. [Google Scholar]
- 54.Yang L, Wang Y, Sun M, et al. Information processing speed and related factors in first-episode schizophreniaJ. Journal of Shandong University 2013;9:100–4. [Google Scholar]
- 55.Zhang Z, Zhou F, He F, et al. Cognitive functionin patients with first-episode schizophrenia and individuals at high-risk for psychosis J. Chinese Mental Health Journal 2017;5:345–9. [Google Scholar]
- 56.Chen J, Jiang C, Tian L, et al. The cognitive function of first-episode schizophrenia patients and healthy first-level relatives of schizophrenic patients. J. Journal of Chinese Physician 2014;12:1659–62. [Google Scholar]
- 57.Ma J, Yu W, Pan Z. The study of first-episode schizophrenia cognitive function. J. Medical Journal of Chinese People's Health 2010;5:526–7. [Google Scholar]
- 58.Wang S, Chen Y, Chen Y, et al. The comparative study of cognitive function damage in first-episode schizophrenia. J. Lab Med Clin 2011;19:2315–6. [Google Scholar]
- 59.Qi J. Comparative study of cognitive function in first-episode schizophrenia and ultra high risk population. D. Xinxiang Medical University 2015. [Google Scholar]
- 60.Chen Q, Qian M, Wang Y, et al. Control study of cognitive function in ultra-high risk population of schizophrenia and first-episode schizophrenia patientsJ. Journal of Tianjin Medical University 2014;3:216–9. [Google Scholar]
- 61.Chan RCK, Chen EYH, Law CW. Specific executive dysfunction in patients with first-episode medication-naïve schizophrenia. Schizophrenia Research 2006;82:51–64. 10.1016/j.schres.2005.09.020 [DOI] [PubMed] [Google Scholar]
- 62.Guo X, Li J, Wang J, et al. Hippocampal and orbital inferior frontal gray matter volume abnormalities and cognitive deficit in treatment-naive, first-episode patients with schizophrenia. Schizophr Res 2014;152:339–43. 10.1016/j.schres.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 63.He Z, Deng W, Li M, et al. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med 2013;43:769–80. 10.1017/S0033291712001638 [DOI] [PubMed] [Google Scholar]
- 64.Hou C-L, Xiang Y-T, Wang Z-L, et al. Cognitive functioning in individuals at ultra-high risk for psychosis, first-degree relatives of patients with psychosis and patients with first-episode schizophrenia. Schizophrenia Research 2016;174:71–6. 10.1016/j.schres.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 65.Hu M, Chen J, Li L, et al. Cognitive functioning of first-episode schizophrenia patients and their healthy siblingsJ. Chin J Psychiatry 2011;4:208–11. [Google Scholar]
- 66.Zhao S, Zhang T, Tang Y, et al. Comparative research between cognitive changes in patients at clinical high risk for psychosis and drug-naive first-episode schizophrenia patientsJ. Journal of Neuroscience and Mental Health 2014;2:130–3. [Google Scholar]
- 67.Zhang Y, Guo X, Zhao J. Cognitive function in first-episode adolescent-onset schizophrenia before and after treatmentJ. Chin J Psychiatry 2015;5:292–6. [Google Scholar]
- 68.Fu W. The study of cognitive function and event related potential in first episode schizophrenia patients. D. Nanchang University 2018. [Google Scholar]
- 69.Yu L. Study on the correlation between MIF, EGF and first-episode schizophrenia. D. Kunming Medical University 2018. [Google Scholar]
- 70.Liang S, Deng W, Wang Q, et al. Performance of verbal fluency as an endophenotype in patients with familial versus sporadic schizophrenia and their parents. Scientific Reports 2016;6 10.1038/srep32597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Yao J, Lv Y, et al. An association study on the cognitive function and the cerebral grey matter volume of patients with First-Episode SchizophreniaJ. Shanghai Arichives of Psychiatry 2018;30:154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu M, Zong X, Tang J, et al. Cognitive function changes with risperidone monotherapy and their relationship to psychotic symptoms in treatment-naive first-episode schizophrenia patients. J Inter Psychiatry 2018;45. [Google Scholar]
- 73.Xing Y, Yang X, Dong Q, et al. Cognitive function of patients with first-onset schizophreniaJ. J Clin Psychol Med 1999;6:334–6. [Google Scholar]
- 74.Ma X, Wang Q, Sun X, et al. Genetic study of neurocognitive function in first-episode schizophreniaJ. Chin J Psychiatry 2004;3:140–4. [Google Scholar]
- 75.Liang Y, Han Y, Song L, et al. A control study of neuropsychological function in 35 schizophrenic patients. J Chin Ment Heal 2008;10:713–6. +728. [Google Scholar]
- 76.Dong X. A comparative study of event-related potential contingent negative variation in first episode schizophrenia. D. Jining Medical University 2018. [Google Scholar]
- 77.Zhou Y, Zhai J, Chen M. Influence of duration of untreated psychosis on cognition and social function in first-episode drug-naive schizophrenia. J. Chin J Nerv Ment Dis 2018;44. [Google Scholar]
- 78.Zhou F-C, Xiang Y-T, Wang C-Y, et al. Characteristics and clinical correlates of prospective memory performance in first-episode schizophrenia. Schizophrenia Research 2012;135:34–9. 10.1016/j.schres.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 79.Zhou F-C, Hou W-M, Wang C-Y, et al. Prospective memory performance in Non-Psychotic first-degree relatives of patients with schizophrenia: a controlled study. PLoS ONE 2014;9 10.1371/journal.pone.0111562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou FC, Wang CY, Ungvari GS, et al. Longitudinal changes in prospective memory and their clinical correlates at 1-year follow-up in first-episode schizophreniaJ. PLoS ONE 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao X. The serum p-mTOR, IL-10 level change and correlation analysis in patients with prominent negative symptoms of schizophrenia. D. Kunming Medical School 2018. [Google Scholar]
- 82.Chan KK, Hui CL, Lam MM, et al. A three-year prospective study of spontaneous eye-blink rate in first-episode schizophrenia: relationship with relapse and neurocognitive function. East Asian Arch Psychiatry 2010;20:174–9. [PubMed] [Google Scholar]
- 83.Ge Y, Zhuang L, Yan P, et al. Study of working memory impairment in first-episode schizophreniaJ. Psychological Doctor 2018;24:31–2. [Google Scholar]
- 84.Li Z, Huang P, Zhang F, et al. Effects of aripiprazole on rehabilitation of cognitive function in first-episode schizophrenia. Chin J of Behavioral Med Sci 2008;46:20–2. [Google Scholar]
- 85.Xiao Y, Fan M, Wu F. Correlation of cognitive function and psychopathological symptoms in schizophrenic super high risk population and intervention effect of Paliperidone. J China Pharmaceuticals 2014;23:7–9. [Google Scholar]
- 86.De Herdt A, Wampers M, Vancampfort D, et al. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: A meta-analysis. Schizophr Res 2013;149:48–55. 10.1016/j.schres.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 87.Hong KS, Kim JG, Koh HJ, et al. Effects of risperidone on information processing and attention in first-episode schizophrenia. Schizophr Res 2002;53:7–16. 10.1016/S0920-9964(01)00167-0 [DOI] [PubMed] [Google Scholar]
- 88.Andersen R, Fagerlund B, Rasmussen H, et al. Cognitive effects of six months of treatment with quetiapine in antipsychotic-naïve first-episode schizophrenia. Psychiatry Res 2011;187:49–54. 10.1016/j.psychres.2010.10.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2018-100043supp001.docx (438.6KB, docx)