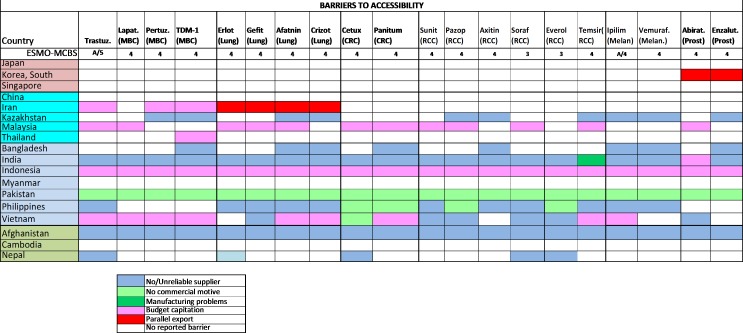
Figure 6.
2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: (1) Asian data of recently approved medications not on the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than two (2): Dominant barrier to accessibility.18 Abirat, abiraterone; Aftatin, atafinib; Axitin, axitinib; Cetux, cetuximab; CRC, colorectal cancer; Erlot, eroltinib; Enzalut, enzalutamide; Everol, everolimus; Gefit, gefitinib; Ipilim, ipilimumab; Lapat, lapatinib; MBC, metastatic breast cancer; Melan, melanoma; Panitum, panitumumab; Pazop, pazopanib; Pertuz, pertuzumab; Prost, prostate; RCC, renal cell cancer; Soraf, sorafenib; Suni, sunitinib; Vemuraf, vemurafenib.Figure reproduced with permission from Cherny et al., Annals of Oncology 2017