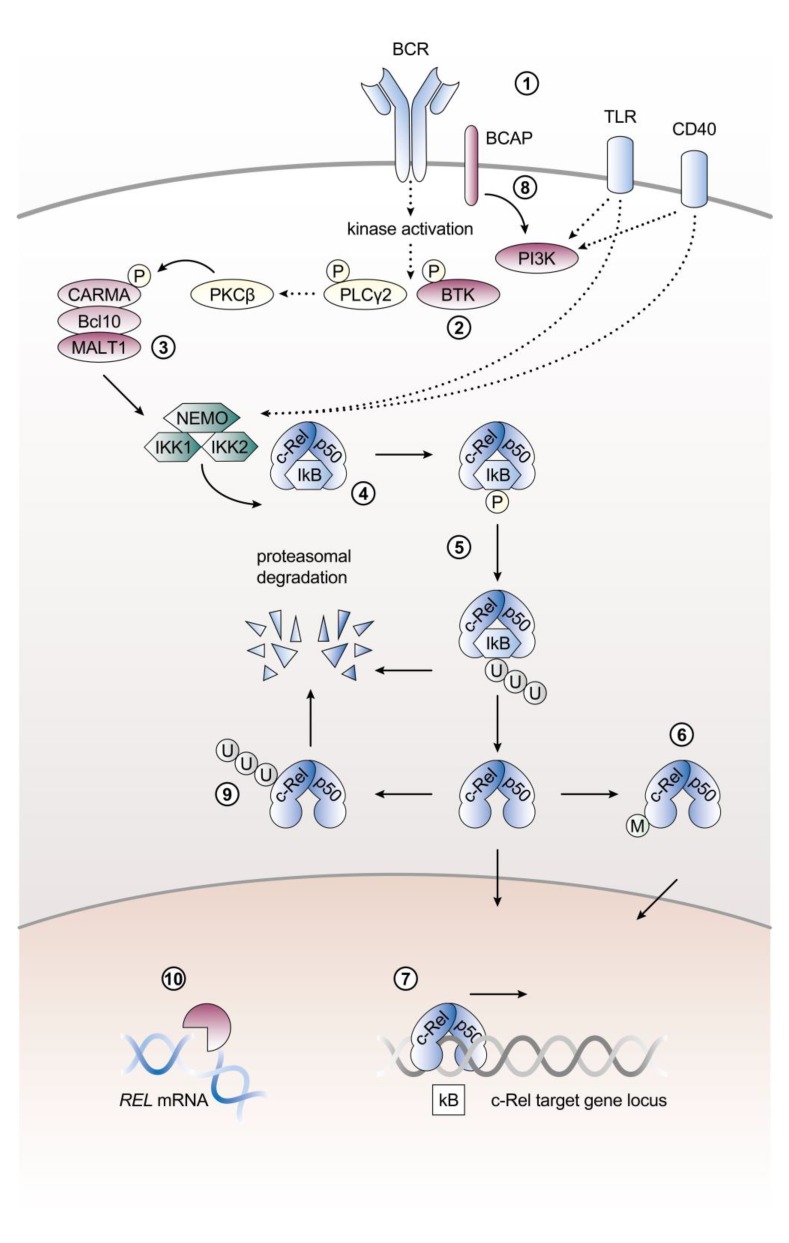
Figure 2.
Activation of c-Rel signaling by the canonical NF-κB pathway in B cells. Major signaling and regulatory components described in the main text are illustrated. (1) Cardinal triggers of c-Rel signaling include B cell receptor (BCR), toll-like receptor (TLR) or CD40 stimulation. (2) In Btk-deficient B cells c-Rel DNA-binding activity is strongly reduced following stimulation. (3) The paracaspase mucosa-associated lymphoid tissue protein 1 (MALT1) which is part of the CBM complex is specifically required for BCR signal-induced c-Rel nuclear translocation. (4) In mature B cells the predominant NF-κB dimers are formed by c-Rel and p50. (5) c-Rel is sequestered in the cytoplasm by interaction with the inhibitory proteins IκBα, IκBβ and IκBε. Various upstream stimuli of canonical NF-κB signaling can target these IκB proteins for proteasomal degradation. (6) c-Rel is modified on a post-translational level. These post-translational modifications (M) can influence c-Rel transactivation and transforming activity. (7) Once released from the inhibitory IκB proteins, c-Rel translocates into the nucleus and binds to κB target sites to exert its function as a transcription factor. (8) PI3K signaling contributes to maintaining c-Rel levels in B cells. (9) Regulation of c-Rel is mediated by ubiquitination and subsequent proteasomal degradation. (10) REL mRNA levels are controlled on a post-transcriptional level. References as well as further details and abbreviations are provided in the main text. In addition to references cited in the main text, the content of this figure is based on Okkenhaug and Vanhaesebroeck, 2003 [65], Siebenlist et al. 2005 [66], and Murphy et al. 2007 [67]. P, phosphorylation; U, ubiquitination; M, post-translational modification.