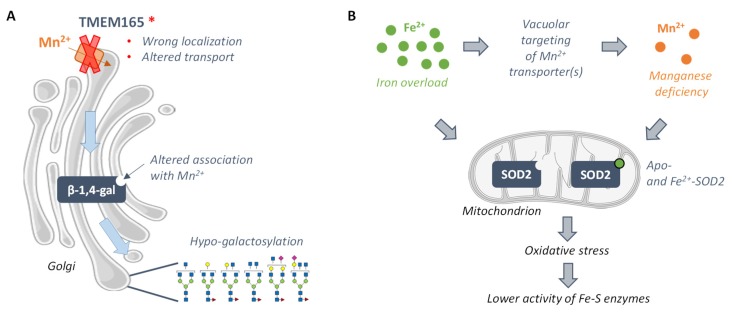
Figure 2.
Manganese-related molecular mechanisms beyond the development of Transmembrane protein 165-Congenital Disorder of Glycosylation (TMEM165-CDG) (A) and of Friedreich ataxia (B). (A) Disease-causing mutations within the human gene coding for the Golgi-localized protein TMEM165 are thought to cause its mislocalization or to affect its transport capacity, both leading to disturbed manganese homeostasis in the Golgi lumen. In this compartment, the altered bioavailability of manganese for the Mn2+-dependent β-1,4-galactosyltransferase (β-1,4-gal) would affect its enzymatic function and lead to the production of hypo-galactosylated N-glycans. (B) Friedreich ataxia is characterized by a decreased production of the mitochondrial frataxin and by iron overload. This metal overaccumulation is, in turn, thought to induce, in yeast, vacuolar targeting of the protein Smf2p, that plays a key role in manganese dispatching within the cell, which, in turn, leads to cellular manganese deficiency. Both manganese deficiency and iron overload drive the formation of the inactive apo- and Fe-bound superoxide dismutase 2 (SOD2), thereby altering oxidative stress protection and leading to decreased activities of the Fe-S enzymes.