|
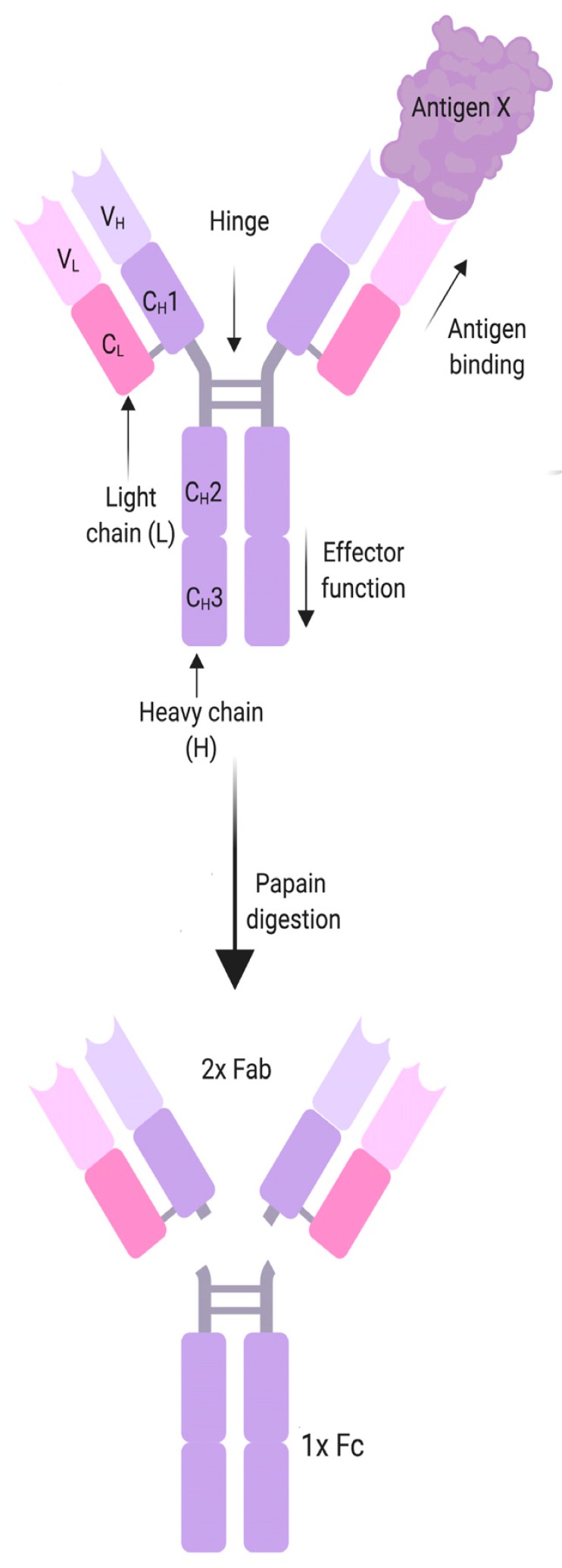
MG antibodies belong to the IgG class, which can also be divided into the Fc and the Fab region. Historically these names derive from the two breakdown products generated when IgG is digested with papain. The “Fab” part, for “Fragment, antigen binding”, is the part of the antibody that contains the variable regions of the heavy and light chains (VL, VH), which binds the antigen, as well as the first constant domain (CH1, CL). Fc stands for “fragment, crystallized”, and comprises most of the constant region of the two heavy chains of an antibody (CH2-CH3), and determines the antibody class and subclass, whether the antibody is membrane bound or soluble, and the effector mechanisms of the antibody. These may include the activation of the classical complement cascade, antibody-dependent cellular cytotoxicity, opsonization, blocking enzymes or receptors or formation of immune complexes. Many autoantibodies belong to the IgG1 and IgG3 subclasses that bind C1q and activate the classical complement pathway, and that also bind to activating Fcγ receptors on immune cells leading to their activation. IgG1, 2 and 3 also cross-link antigens, forming either immune complexes with soluble antigen, or causing endocytosis of transmembrane proteins. In recent years a range of autoantibodies associated with IgG4 subclass antibodies were discovered. Due to structural differences in the Fc region, IgG4 does not bind C1q or activating Fcγ receptors and is bi-specific, and therefore has an “anti-inflammatory” effect thought to counteract overshooting immune responses after chronic antigen exposure. IgG4 autoantibodies are usually pathogenic by a blocking mechanism that affects enzyme or receptor function or disrupts protein-protein interaction. Figure created with BioRender. |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.