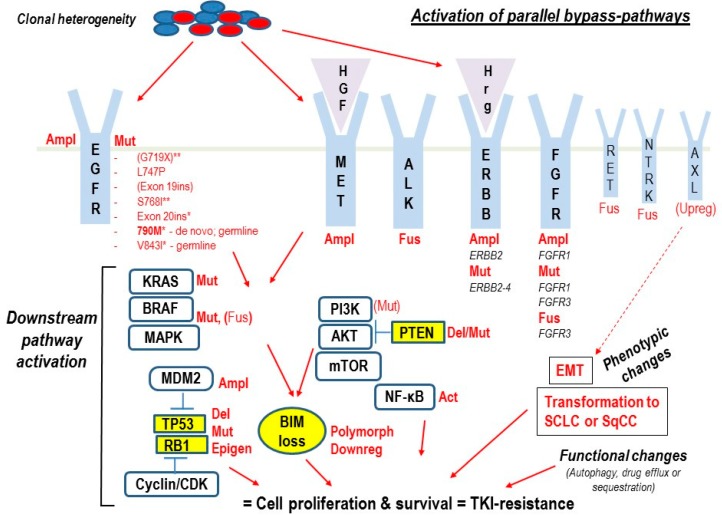
Figure 1.
Summary of main molecular mechanisms of intrinsic resistance to EGFR-tyrosine-kinase-inhibitors (EGFR-TKIs) in EGFR-mutated (EGFRM+) non-small cell lung cancer (NSCLC). Given the clonal genetic heterogeneity of NSCLC, innate genetic alterations capable of impairing the response and causing intrinsic resistance to TKIs may be present in pre-existing clones before treatment (de novo alterations) or may be very rapidly induced in surviving cancer cells as immediate adaptive response or tolerance to the targeted therapy. If the relative allelic frequency of one or several (polyclonal resistance) of these pre-existing/immediately induced alterations is sufficient to very rapidly counteract the effect of TKIs (conventionally within the first 3 months after TKI-treatment initiation), tumor cells will continue to proliferate and survive, and intrinsic TKI-resistance will ensue. The EGFR-dependent resistance mechanisms are represented by amplification (Ampl) and/or specific somatic or germline mutations (Mut) of the EGFR-gene. Some of these mutations cause resistance to EGFR-TKIs of all three generations, while others are sensitive to 2G or 3G TKIs, as indicated (* = EGFR-mutants resistant to 1G/2G EGFR-TKIs, but sensitive to 3G TKIs, the most common being T790M indicated in bold; ** = EGFR-mutants resistant to 1G EGFR-TKIs, but sensitive to afatinib). Among the uncommon EGFR-mutations G719X and insertions in exon 19 (Exon 19ins) are indicated in brackets, because despite being less sensitive than common EGFR-mutants, they may show some response to 1G TKIs. Instead, most of the EGFR-independent resistance mechanisms are shared by EGFR-TKIs of all three generations and include the activation of by-pass pathways via amplification (Ampl), mutation (Mut) or fusion (Fus) of alternative parallel receptor tyrosine kinase- (RTK)-genes such as Mesenchymal-Epithelial Transition (MET), Anaplastic Lymphoma Kinase (ALK), non-EGFR Erythroblastic Oncogene B(ERBB)-family-members, Fibroblast Growth Factor Receptor genes (FGFRs) (written in bold), and possibly REarranged during Transfection (RET) and Neurotrophic Tyrosine Receptor Kinase (NTRK) (not in bold). Activation of parallel RTKs can also be induced by overexpression of hepatocyte growth factor (HGF) that binds the MET-receptor or Heregulin (Hrg) that binds ERBB2. Alternative downstream by-pass mechanisms of resistance are represented by mutations, fusions, or deletion (Del) of members of the RAS-RAF-MEK-MAPK and PI3K-AKT-PTEN-mTOR pathways or inactivation of TP53 and/or retinoblastoma 1 (RB1) tumor-suppressor genes via mutation/deletion/epigenetic mechanism (Epigen) or indirectly by gene-amplification of the p53-inhibitor Mouse Double Minute 2 homolog (MDM2) and mutation/amplification of genes encoding cyclins and cyclin-dependent kinases (CDKs). Additional by-pass mechanisms are activation (Act) of the NF-κB transcription factor by different pathways or impairment of TKI-induced apoptosis by loss of the pro-apoptotic BIM-gene expression due to genetic polymorphism (Polym) or transcriptional downregulation (Downreg). Putative mechanisms of intrinsic (and acquired) resistance to all three generations’ TKIs that await further clinical validation are phenotypic changes, such as epithelial-mesenchymal transition (EMT), transformation to small-cell lung cancer (SCLC) or squamous cell carcinomas (SqCC), and potential functional changes reducing TKI efficacy, like rapidly increased autophagic activity, drug-efflux or intracellular drug-sequestration in cancer cells. Some evidence for NSCLC cases with pre-existing, inherently TKI-resistant cells due to upregulation (Upreg; in brackets) of the EMT-inducing RTK AXL has also been provided. RTKs are in light blue, intracellular downstream oncoproteins in white boxes, tumor suppressors in yellow symbols.