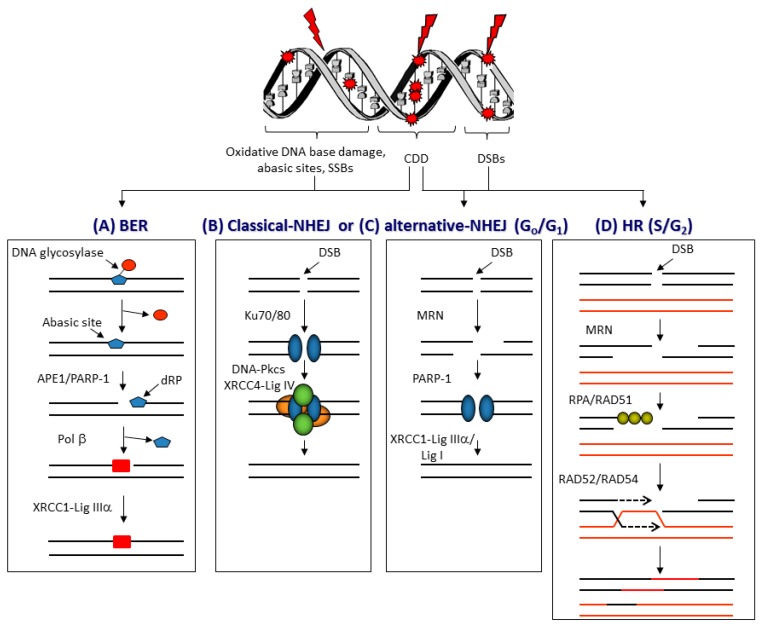
Figure 2.
The response to ionising radiation (IR)-induced DNA damage. Proton beam therapy (PBT), similar to other radiotherapy techniques, targets DNA and can generate an abundance of DNA lesions, where oxidative DNA base damage, abasic sites, and single-strand breaks (SSBs) predominate, and which are repaired via (A) the base excision repair (BER) pathway. This involves recognition of the damaged base by a damage specific DNA glycosylase, incision of the abasic site by AP-endonuclease 1 (APE1) and SSB binding by poly(ADP-ribose) polymerase-1 (PARP-1), 5’-deoxyribosephosphate (dRP) removal and gap filling by DNA polymerase β (Pol β), and finally ligation by X-ray repair cross-complementing protein 1-DNA ligase IIIα (XRCC1–Lig IIIα) complex. Double-strand breaks (DSBs) are repaired by different pathways dependent on cell-cycle phase. In the G0/G1 phases, DSBs are repaired by either (B) classical non-homologous end-joining (NHEJ) involving Ku70/80 that binds to the DNA ends, followed by DNA-dependent protein kinase catalytic subunit (DNA-Pkcs) and XRCC4–Lig IV that promote DNA ligation, or via (C) alternative NHEJ which involves DSB end resection by the MRE11–RAD50–NBS1 (MRN) complex, PARP-1 binding to the DSB ends, and subsequent repair by Lig I or XRCC1–Lig IIIα. In the S/G2 phases of the cell cycle, DSB repair is achieved by (D) homologous recombination (HR) which uses a sister chromatid for repair. Therefore, following DNA end resection by the MRN complex, replication protein A (RPA) and RAD51 bind to the single-stranded DNA overhangs that promote strand invasion and subsequent DNA synthesis in the presence of RAD52/RAD54, as well as formation and resolving of Holliday junctions. The induction of complex DNA damage (CDD), consisting of several DNA lesions in close proximity, particularly by high-LET protons at the distal edge of the SOBP, likely require multiple pathways for repair.