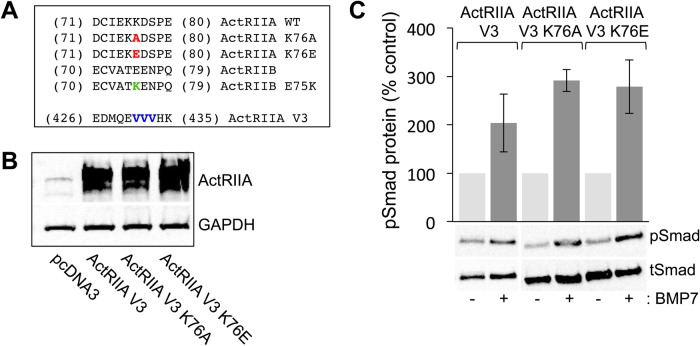
Fig. 4.
Site-directed mutagenesis of K76 in ActRIIA does not impede variant receptor expression or BMP7-stimulated Smad1/5/8 phosphorylation. (A) Amino acid sequences of mouse ActRIIA in the region of the K76 (red) and V3 (blue) mutations and mouse ActRIIB in the region of the E75 (green) mutation. Numbering corresponds to the amino acid number: for the K76 and E75 mutations according to PDB ID: 1LX5 and for the V3 mutations according to NCBI accession no.: NP_031422.3 (Fig. S3). The mutations at position 76 in ActRIIA replace lysine (K) with either alanine (A) or glutamic acid (E). The mutation at position 75 in ActRIIB replaces glutamic acid with lysine. The mutations at positions 431 to 433 do not replace the three valine (V) residues in the amino acid sequence but rather alter the nucleotide sequence to create an ActRIIA cDNA that is resistant to sh-AIIA while maintaining the amino acid sequence. (B) Western blots of whole-cell C2C12 lysates, transfected with pcDNA3, ActRIIA V3, ActRIIA V3 K76A or ActRIIA V3 K76E, were probed with an anti-ActRIIA antibody. Detection of GAPDH provided a loading control. (C) Quantification of western blots of transfected whole-cell C2C12 lysates incubated with or without 50 ng/ml BMP7 probed with a phospho-specific Smad1/5/8 antibody. Detection of total Smad1 provided a loading control. Densitometric analysis (mean±s.e.m.; n=2) shows an increase in response to BMP7 in cells expressing ActRIIA V3 (104% over control), ActRIIA V3 K76A (191% over control) and ActRIIA V3 K76E (179% over control).