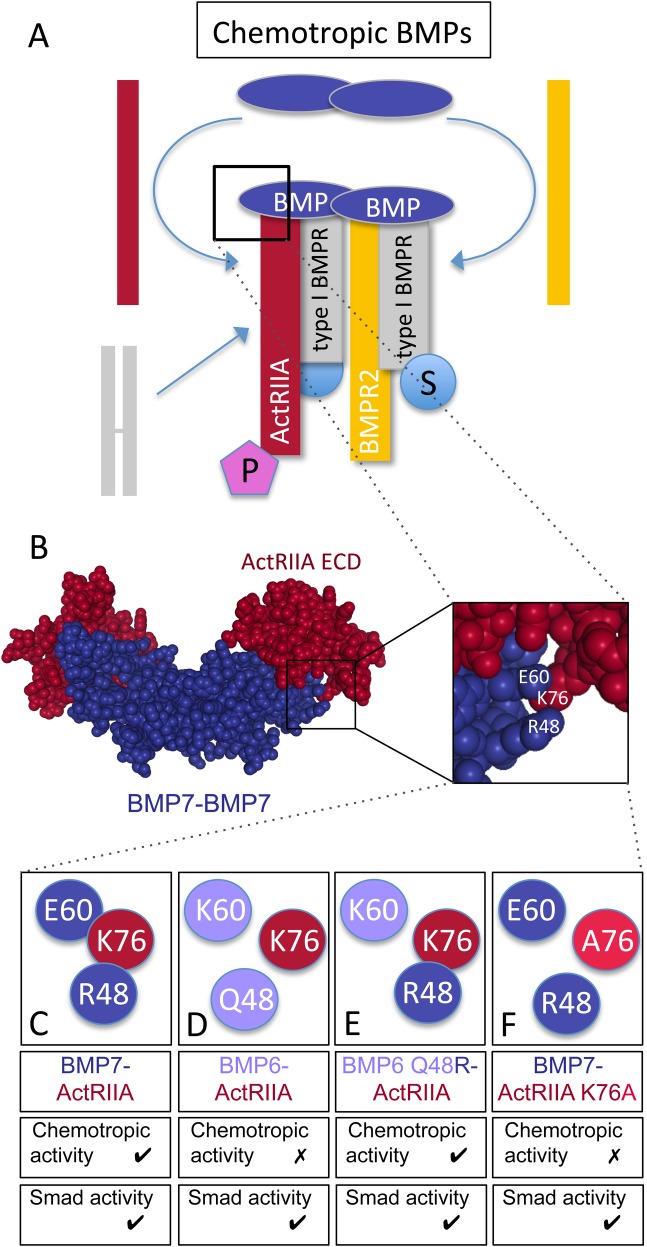
Fig. 8.
Signaling and receptor binding interactions of chemotropic BMPs. (A) Model of chemotropic BMP signaling indicating the potential asymmetric recruitment of ActRIIA (red) and BMPR2 (yellow) into the tetrameric receptor complex. A type I BMP receptor pair is represented as a preformed complex (PFC, gray subunits), though it is unclear whether BMP7 selectively engages PFCs containing ActRIIA and BMPR2 or recruits individual receptors into a complex with type I BMP receptor subunits. BMP7 stimulates Smad- (S) and PI3K-dependent (P) downstream signaling linked to type I and type II BMP receptors, respectively. (B) Spacefill representation of the crystal structure of a BMP7 dimer (blue) bound to the ActRIIA ECD (red) generated from Protein Data Bank ID, 1LX5 (Greenwald et al., 2003) using NGL Viewer (Rose et al., 2018). Enlarged area indicates amino acid positions for BMP7 R48 and E60 as well as ActRIIA K76. (C–F) Representations of wild-type and mutated amino acids in BMP-ActRIIA interactions and the associated chemotropic and Smad activity. (C) BMP7 R48 is predicted to associate with ActRIIA K76 and BMP7-evoked chemotropic activity requires interaction with ActRIIA. (D) BMP6 Q48 is not predicted to associate with ActRIIA K76 and BMP6 does not stimulate chemotropic activity. (E) BMP6 Q48R demonstrates potent chemotropic activity and would potentially interact with ActRIIA K76. (F) Mutation of K76 in ActRIIA to alanine (A76) is predicted to disrupt the interaction with BMP7 R48. The presence of ActRIIA K76A blocks BMP7-evoked chemotropic activity. Mutations at this site do not have any effect on BMP-stimulated Smad phosphorylation.